Abstract
A fatal case of primary amebic meningoencephalitis (PAM) in a 5-month-old infant is described. The disease may have been contracted during bathing. The source of water was from an artificial well. The clinical presentation, the isolation of the ameba from the cerebrospinal fluid, the poor response to amphotericin B, and the ultimate fatal outcome are all consistent with the diagnosis of PAM. On the basis of its ability to grow at temperatures above 30°C, the morphology of the trophozoite, and the presence of flagellate forms, the ameba was identified as Naegleria fowleri. Pathogenic N. fowleri amebae were recovered from samples of water from the well. To our knowledge this case represents the second case of PAM in an infant in the absence of the history of swimming.
Naegleria fowleri, a free-living ameba, is ubiquitous and worldwide in distribution. It can infect humans and cause significant disease. It penetrates through the cribriform plate and can cause fulminant and rapidly fatal meningoencephalitis. Most of the infections have been associated with bathing in streams, ponds or lakes, and indoor swimming baths where the water is warm (7). We report on a case of primary amebic meningoencephalitis (PAM) in an infant who had not been swimming. To our knowledge this is the first case of PAM to be reported in Mangalore, India.
Case history.
A 5-month-old infant presented with a history of fever, vomiting for 1 week, and convulsions for 3 days. On physical examination, the child was febrile (38.5°C), continuous convulsions were present, and the pupils were reactive. There was a progressive deterioration of consciousness, leading to coma. There was no significant finding in the cardiovascular system or respiratory system or by abdominal examination. A computed tomography scan showed mild effacement of the basal cisterns and a thin hypodense collection in bilateral frontal convexity, suggesting a possibility of subdural hygroma or subdural effusion.
Laboratory data included a total leukocyte count of 7,300/mm3, with 71% neutrophils, 26% lymphocytes, and 3% eosinophils. The hemoglobin concentration was 10.2 g/dl. No parasites were seen in the peripheral smear. The blood electrolyte picture showed sodium at 133 mmol/liter, potassium at 4.4 mmol/liter, chloride at 95 mmol/liter, bicarbonate at 18.6 mmol/liter, phosphorus at 4.7 mg/dl, blood urea nitrogen at 14 mg/dl, a serum calcium level of 9.2 mg%, and a random blood sugar level of 120 mg/dl.
The cerebrospinal fluid (CSF) was cloudy and hemorrhagic; and analysis showed the presence of sugar at a concentration of 10 mg/dl, proteins at a concentration of 361 mg/dl, and a cell count of 130 cells/mm3, with 80% neutrophils and 20% lymphocytes. Gram staining showed numerous polymorphonuclear leukocytes but no bacteria. Examination of a wet mount showed actively motile trophozoites suggestive of PAM. A Giemsa-stained smear showed trophozoites.
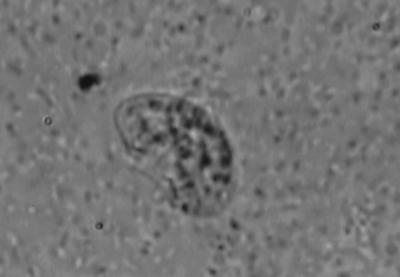
CSF sediment culture on 1.5% nonnutrient agar preseeded with a lawn culture of Escherichia coli yielded oval, motile, flagellated forms of Naegleria fowleri (length, about 15 μm) after 48 h of incubation, as observed in a wet mount preparation (Fig. 1). Bacteriological culture yielded no growth after 48 h of incubation.
FIG. 1.
Flagellated form of N. fowleri.
N. fowleri was isolated from well water, which was used to bathe the child. The well water was not chlorinated.
Amphotericin B (0.6 mg/kg of body weight) was given intravenously, and ceftriazone was given at 100 mg/kg of body weight. There was progressive deterioration of consciousness and a nonreactive pupillary reaction, leading to coma, and the child died on the 2nd day of admission.
DISCUSSION
N. fowleri is distributed worldwide in thermally polluted streams and tolerates temperatures of 40 to 45°C (4). The amebae can also be found in coastal water, freshwater, sewage, heating and ventilation units, poorly chlorinated swimming pools, artificial lakes, and warm water near the discharge outlets of power plants (5).
In Australia, one fatal case of PAM led to the detection of Naegleria spp. in the household water supply. This emphasizes the fact that the infection may be associated with washing and bathing as well as with swimming (3). In PAM, infection occurs via the olfactory tract, and infection of the brain is direct; hematogenous spread does not occur in humans. In a young child in northern Nigeria, there was no association with swimming in water and death that occurred from PAM due to N. fowleri (2).
To our knowledge, the case described here represents the second case of PAM in an infant without a history of swimming. N. fowleri could have entered the patient through the nose while the child was being bathed. The water used for bathing may have been the source of infection. Warm water temperatures (above 30°C) and pollution of the water with organic material are ideal for the proliferation of N. fowleri (6). Since N. fowleri is susceptible to chlorine at 2 ppm, it is necessary that wells be properly maintained with adequate chlorination.
The differential diagnosis of PAM includes bacterial meningitis. Suspicion of PAM should be aroused when the CSF is purulent with no bacteria. In such cases, a wet drop preparation should be made. The CSF should not be centrifuged or refrigerated. The amebae are easily seen and recognized by their slug-like movement (7).
The results of treatment on the whole have been disappointing. The only drug that appears to be promising against N. fowleri in vivo is amphotericin B at a dose of 1 mg/kg/day administered intravenously as a slow infusion or in combination with 0.7 mg of amphotericin B intrathecally on alternate days. Only a few cases of successful treatment have been reported so far (1).
REFERENCES
- 1.Cerva, L. 1986. Amoebic meningoencephalitis, p.1095–1098. In A. I. Braude, C. E. Davis, and J. Fierer (ed.), Infectious diseases and medical microbiology, 2nd ed. The W. B. Saunders Co., Philadelphia, Pa.
- 2.Lawande, R. V., I. John, R. H. Dobbs, and L. J. Egler. 1979. A case of primary amebic meningoencephalitis in Zaria, Nigeria. Am. J. Clin. Pathol. 71:591–594. [DOI] [PubMed] [Google Scholar]
- 3.Marciano-Cabral, F. 1988. Biology of Naegleria spp. Microbiol. Rev. 52:114–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall, M. M., D. Naumovitz, Y. Ortega, and C. R. Sterling. 1997. Waterborne protozoan pathogens. Clin. Microbiol. Rev. 10:67–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez, A. J. 1993. Free-living amebas: infection of the central nervous system. Mt. Sinai J. Med. 60:271–278. [PubMed] [Google Scholar]
- 6.Martinez, A. J., and G. S. Visvesvara. 1999. Free-living amebae: Naegleria, Acanthamoeba and Balamuthia infections, p.814–824. In R. C. Guerrant, D. H. Walker, and P. F. Weller (ed.), Tropical infectious diseases: principles, pathogens and practice. Churchill Livingstone, Philadelphia, Pa.
- 7.Warhurst, D. C. 1985. Water: infection caused by free living amoebae, p.572–574. In P. E. C. Manson-Bahr and D. R. Bell (ed.), Manson’s tropical diseases, 19th ed. ELBS Balliere Tindall, London, United Kingdom.