Fig. 1. Topology of human CTR1 and highly conserved Gly-X-X-X-Gly motif.
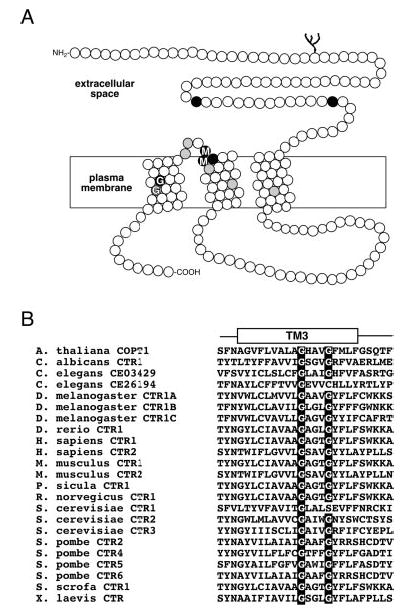
A, diagram of a human CTR1 monomer shows the extracellular N terminus, three putative transmembrane domains, and an intracellular C terminus. Amino acid positions represented as black circles are invariantly conserved in CTRs from yeast, plants, and metazoans, and those in gray are >90% identical in the family. Two conserved amino acid motifs in the entire family of CTR copper uptake proteins, Met-X-X-X-Met in TM2 and Gly-X-X-X-Gly (GG4) motif in TM3, are indicated. B, multiple amino acid sequence alignment of the third TM domain reveals a nearly invariant conservation of the GG4 motif in all of the known members of the CTR copper uptake family.
