Fig. 5. Processing of GG4 mutants and induction of the unfolded protein response.
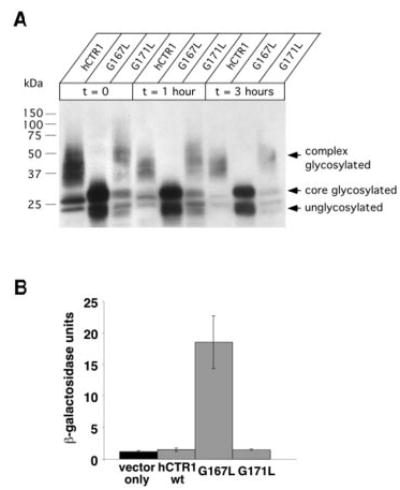
A, yeast cultures expressing the indicated constructs were grown to mid-log phase before protein synthesis was halted with cycloheximide. Samples were removed from the culture at the indicated time points, and total protein extracts were subjected to Western blot using an anti-HA primary antibody. Note that a nearly overexposed blot demonstrates the lack of any higher order glycosylation for the G167L mutant compared with wild type and G171L. The doublet of bands observed for the G167L mutant corresponds to unglycosylated and core-glycosylated species, respectively. Recall in Fig. 4 that complex glycosylation (seen as high molecular mass smearing) as well as core glycosylation (as a distinct band that migrated at ~28 kDa), could be reduced to a single band (~23 kDa) upon exposure to PNGase F. B, yeast transformed with both pSZ1-UPRE-LacZ and an expression plasmid carrying either wild type G167L or G171L were subjected to β-galactosidase assay to determine the extent of induction of the unfolded protein response (n = 3–4 randomly selected colonies/condition).
