Fig. 1. Effect of caldesmon on the actin-activated ATPase activity of skeletal muscle papain (Mg2+) S-1 and the binding of skeletal muscle papain (Mg2+) S-1·ATP to actin-tropomyosin.
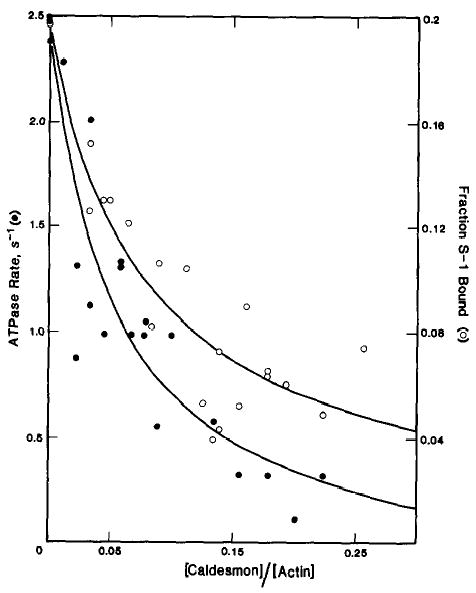
ATPase rates (•) and binding (○) were measured in the presence of 50 μm skeletal muscle actin, 10 μm smooth muscle tropomyosin, and 0.2 μm skeletal muscle papain (Mg2+) S-1. The conditions used were 42 mm NaCl, 4.2 mm MgCl2, 10 mm imidazole-HCl (pH 7), 1 mm dithiothreitol, 2 mm ATP, at 25 °C. The ATPase activity in the absence of caldesmon was 2.5 s−1. The fraction of skeletal muscle papain (Mg2+) S-1 bound in the absence of caldesmon was 0.2.
