Abstract
While investigating the requirement for phagosomal alkalinization in the host defense against pulmonary aspergillosis, we observed high morbidity of p47phox−/− mice infected with pH-insensitive Aspergillus nidulans mutants despite a paucity of fungal growth. Fatal infection also resulted from a normally avirulent p-aminobenzoate auxotroph. This demonstrates that p47phox−/− murine immunity contributes significantly to A. nidulans lethality. These data have wider implications for microbial virulence studies with p47phox−/− mice.
The elimination of invading microbes by professional phagocytes is crucial to mammalian immunity. An essential role for the phagocyte NADPH oxidase enzyme complex in this process has been well established from in vitro studies (2, 12, 13, 22) and the human condition chronic granulomatous disorder (CGD) (14, 16, 18). CGD polymorphonuclear leukocytes (PMNs) fail to alkalinize following phagocytosis (5, 15). Targeted deletion of murine p47phox (6) or gp91phox (11) NADPH oxidase subunits mimics CGD and results in susceptibility to Staphylococcus aureus (6, 11) and Aspergillus fumigatus (11) infection characterized by persistent granulomatous inflammation (11, 6).
We recently characterized the role of Aspergillus nidulans pH signal transduction in virulence, finding that mutants lacking the processed form of the pH-responsive transcription factor PacC (1, 19) have alkali-sensitive phenotypes and are dramatically attenuated in neutropenic mice (3). A plausible role for PacC during pathogenesis is the mediation of adaptation to phagolysosomal alkalinization. Such adaptation should be dispensable in CGD, as NADPH deficient PMNs have abnormally acidic phagolysosomes (5, 15).
We tested this hypothesis using p47phox−/− mice and A. nidulans pH response mutants C209 and C14 (3), which, as a consequence of pacC mutations, have non-alkaline-responsive and alkaline-adapted phenotypes, respectively. Murine infection regimens and histopathological analyses were as previously described (3) except that neither p47phox−/− nor parental 129sv mice were chemotherapeutically immunosuppressed. A 20% reduction in body weight measured from the day of infection was used as a surrogate marker of survival, at which point mice were sacrificed. This coincided with emergence of other indicators of severe infection, such as hunched posture, labored breathing, or a moribund state. Log rank analysis was performed to test the significance of virulence data, using Prism3 software. In survival comparisons (n = 10) following infection with 106 A. nidulans wild-type (EBPN17) (3) C14, or C209 conidiospores, neither C209 (P = 0.53) nor C14 (P = 0.53) differed significantly from the wild type (data not shown), demonstrating that alkaline adaptation is dispensable for A. nidulans virulence in p47phox−/− mice. There was 100% survival in parental 129sv mice receiving 106 A. nidulans EBPN17 conidiospores (3) and in p47phox−/− mice receiving saline.
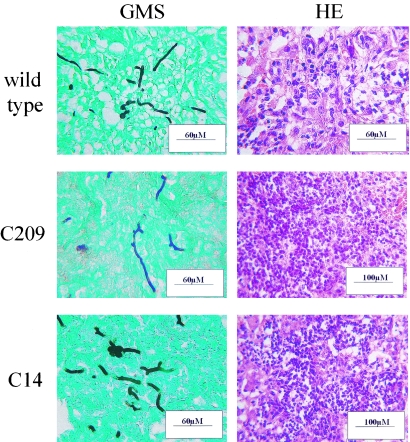
We were unable to detect any differences between A. nidulans wild-type-, C209-, or C14-induced pulmonary histopathology at any infectious dose tested. In a time course experiment we examined two mice each with EBPN17, C209, and C14 at each of three time points: 24, 48, and 72 h. Representative samples from the 48-h time point are shown in Fig. 1. In a separate experiment using 104 conidiospores (in which most mice survived), we examined four mice for each of the three strains at 14 days postinfection (data not shown). In addition, we examined multiple samples from unmatched time points between 17 h and 14 days postinfection for all three strains (data not shown). For every lung studied histopathologically, we examined 32 slides, derived by quadruple sampling (for each of two staining treatments) at each of four lateral sectioning levels. At 48 h postinfection, consolidated inflammation was evident across large regions of lung tissue and mass migration of PMNs into the lung parenchyma prevented the visualization of pulmonary architecture (Fig. 1). Grocott's methanamine silver (GMS) staining revealed the presence of numerous, evenly distributed hyphal elements, some of which had branched. Throughout the study hyphal growth in vivo was independent of the genotype of the infecting strain. These findings indicate that, unlike in neutropenic murine infection (3), PacC is not required for A. nidulans virulence in p47phox−/− mice.
FIG. 1.
A. nidulans pH mutants cause disease similar to that caused by the wild type in p47phox−/− mice. Wild-type, alkaline-nonresponsive, and alkaline-adapted A. nidulans strains show similar histopathological features of disease progression in p47phox−/− mice. Lungs were removed 48 h after infection with 106 A. nidulans conidiospores of the wild-type, alkaline-nonresponsive (C209), and alkaline-adapted (C14) strains. GMS-stained sections reveal equivalent fungal burdens, while hematoxylin-and-eosin (HE) staining shows a massive influx of PMNs.
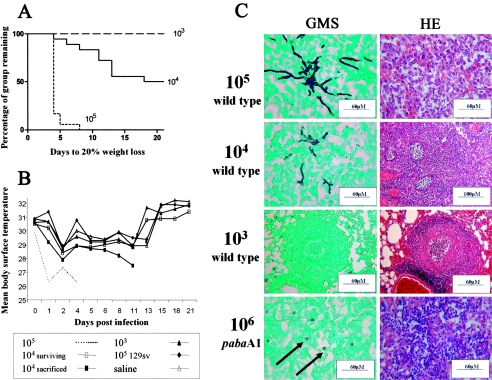
A. fumigatus infection of gp91phox−/− mice causes abnormal inflammatory provocation, even following inoculation with sterile hyphae (9). To determine the contribution of the aberrant inflammatory response (Fig. 1) to experimental outcome in our model, we examined wild-type A. nidulans disease progression and infection characteristics (Fig. 2). p47phox−/− murine weight loss and body temperature reductions (n = 18) were dramatic following infection with 105 EBPN17 (3) spores (Fig. 2A and B). Extensive bronchopneumonia characterized by large areas of neutrophil-saturated tissue was evident by 48 h postinfection, and the majority of animals in this group had been culled by day 4 (Fig. 2C). Infection with 104 conidiospores was less severe, with 50% of mice surviving to day 21 despite the formation of numerous granulomatous lesions consisting of neutrophils surrounded by epithelioid mononuclear cells (Fig. 2A to C). Although infection with 103 spores was nonfatal, pulmonary tissue granulomas formed throughout the lung parenchyma (Fig. 2A to C).
FIG. 2.
Characterization of pulmonary aspergillosis in p47phox−/− mice. (A) Time to 20% weight loss of p47phox−/− mice following intranasal infection with 105, 104, or 103 A. nidulans wild-type conidiospores. (B) Mean body temperature (°C) of p47phox−/− mice following A. nidulans wild-type infection with 105, 104 (fatal infection and nonfatal infection), and 103 conidiospores. 129sv parental wild-type mice infected with 105 A. nidulans wild-type conidiospores and saline-inoculated p47phox−/− mice served as controls. (C) Histopathological analysis of pulmonary aspergillosis in p47phox−/− mice receiving 105 or 104 A. nidulans wild-type conidiospores at day 4 postinfection, 103 A. nidulans wild-type conidiospores at day 21 postinfection, or 106 A. nidulans p-aminobenzoate auxotroph 302 (pabaA1) conidiospores at day 4 postinfection. Arrows indicate positioning of ungerminated conidiospores.
To characterize further the role of the p47phox−/− immune response to the experimental outcome of A. nidulans infection, we studied the pathogenicity of an attenuated A. nidulans p-aminobenzoate auxotroph, strain 302, which is unable to germinate and grow in vivo (17). To avoid possible ambiguity with a sublethal inoculum, we used an infectious dose expected to result in 100% mortality, as found for the wild-type isolate EBPN17 (3). By day 6 all mice (n = 5) receiving 106 strain 302 conidia had been culled. Histological examination of infected lungs revealed a degree of pulmonary inflammation similar to that induced by an inoculum of 106 wild-type, C209, or C14 conidia (Fig. 1 and 2C). However, GMS staining revealed the absence, in mice culled at 4 and 5 days postinfection, of A. nidulans germination (Fig. 2C), demonstrating that fatal infection in p47phox−/− mice is possible in the absence of fungal growth and supporting a role for aberrant inflammation in murine lethality in this model.
Virulence of normally attenuated mutants in NADPH oxidase-deficient mice has a precedent. Several Salmonella enterica serovar Typhimurium comparative virulence studies (4, 8, 10, 20, 21) have reported that NADPH oxidase-deficient mice have acute sensitivity to S. enterica serovar Typhimurium challenge compared to mice with fully competent respiratory burst activity. Some interpretations of such data attribute resistance to the phagocyte respiratory burst to the gene under investigation. However, our data demonstrate that, in whole-animal virulence studies, the complex inflammatory consequences of NADPH oxidase dysfunction seriously confound comparative virulence studies, which therefore require careful histopathological examination, cautious interpretation, and appropriate controls, including, where possible, an appropriately attenuated mutant. gp91phox−/− and p47phox−/− survival studies previously conducted in the absence of such controls or supporting data (such as those derived from cellular assays) may therefore require reevaluation. Infection using sublethal inocula in p47phox−/− mice may be one means of identifying differences in virulence or host response between strains. We were unable, at sublethal doses, to distinguish any differences between A. nidulans wild-type, C209, or C14 infection in p47phox−/− mice (data not shown).
The majority of the research into the diagnosis, pathology, and treatment of Aspergillus infection has used neutropenic mice (7). The extrapolation of data obtained in this way to the human condition of CGD may be of limited value in terms of improving prospects for this clinical cohort.
Acknowledgments
This work was supported by the Chronic Granulomatous Disorder Research Trust (grant J4G/99/05 to T.R., K.H., and H.N.A.), The Wellcome Trust (grant 067878 to H.N.A.), and the Biotechnological and Biological Sciences Research Council (grant 60/P17835 to H.N.A., K.H., and T.R.).
We thank Mariana Canedo, Lily Stanton, and Phil Muckett for technical assistance and Tony Segal for p47phox−/− mice.
Editor: T. R. Kozel
REFERENCES
- 1.Arst, H. N., Jr., and M. A. Peñalva. 2003. pH regulation in Aspergillus and parallels with higher eukaryotic regulatory systems. Trends Genet. 19:224-231. [DOI] [PubMed] [Google Scholar]
- 2.Babior, B. M. 1999. NADPH oxidase: an update. Blood 93:1464-1476. [PubMed] [Google Scholar]
- 3.Bignell, E., S. Negrete-Urtasun, A. M. Calcagno, H. N. Arst, Jr., K. Haynes, and T. Rogers. 2005. The Aspergillus pH-responsive transcription factor PacC regulates virulence. Mol. Microbiol. 55:1072-1084. [DOI] [PubMed] [Google Scholar]
- 4.De Groote, M. A., U. A. Ochsner, M. U. Shiloh, C. Nathan, J. M. McCord, M. C. Dinauer, S. J. Libby, A. Vazquez-Torres, Y. Xu, and F. C. Fang. 1997. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. USA 94:13997-14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dri, P., G. Presani, S. Perticarari, L. Alberi, M. Prodan, and E. Decleva. 2002. Measurement of phagosomal pH of normal and CGD-like human neutrophils by dual fluorescence flow cytometry. Cytometry 48:159-166. [DOI] [PubMed] [Google Scholar]
- 6.Jackson, S. H., J. I. Gallin, and S. M. Holland. 1995. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 182:751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latgé, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundberg, B. E., R. E. Wolf, Jr., M. C. Dinauer, Y. Xu, and F. C. Fang. 1999. Glucose 6-phosphate dehydrogenase is required for Salmonella typhimurium virulence and resistance to reactive oxygen and nitrogen intermediates. Infect. Immun. 67:436-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgenstern, D. E., M. A. Gifford, L. L. Li, C. M. Doerschuk, and M. C. Dinauer. 1997. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J. Exp. Med. 185:207-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutunga, M., S. Graham, R. D. De Hormaeche, J. A. Musson, J. H. Robinson, P. Mastroeni, C. M. Khan, and C. E. Hormaeche. 2004. Attenuated Salmonella typhimurium htrA mutants cause fatal infections in mice deficient in NADPH oxidase and destroy NADPH oxidase-deficient macrophage monolayers. Vaccine 22:4124-4131. [DOI] [PubMed] [Google Scholar]
- 11.Pollock, J. D., D. A. Williams, M. A. Gifford, L. L. Li, X. Du, J. Fisherman, S. H. Orkin, C. M. Doerschuk, and M. C. Dinauer. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9:202-209. [DOI] [PubMed] [Google Scholar]
- 12.Reeves, E. P., H. Lu, H. L. Jacobs, C. G. Messina, S. Bolsover, G. Gabella, E. O. Potma, A. Warley, J. Roes, and A. W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291-297. [DOI] [PubMed] [Google Scholar]
- 13.Roos, D., R. van Bruggen, and C. Meischl. 2003. Oxidative killing of microbes by neutrophils. Microbes Infect. 5:1307-1315. [DOI] [PubMed] [Google Scholar]
- 14.Segal, A. W. 1996. The NADPH oxidase and chronic granulomatous disease. Mol. Med. 2:129-135. [DOI] [PubMed] [Google Scholar]
- 15.Segal, A. W., M. Geisow, R. Garcia, A. Harper, and R. Miller. 1981. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature 290:406-409. [DOI] [PubMed] [Google Scholar]
- 16.Segal, B. H., T. L. Leto, J. I. Gallin, H. L. Malech, and S. M. Holland. 2000. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore). 79:170-200. [DOI] [PubMed] [Google Scholar]
- 17.Tang, C. M., J. M. Smith, H. N. Arst, Jr., and D. W. Holden. 1994. Virulence studies of Aspergillus nidulans mutants requiring lysine or p-aminobenzoic acid in invasive pulmonary aspergillosis. Infect. Immun. 62:5255-5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thrasher, A. J., N. H. Keep, F. Wientjes, and A. W. Segal. 1994. Chronic granulomatous disease. Biochim. Biophys. Acta 1227:1-24. [DOI] [PubMed] [Google Scholar]
- 19.Tilburn, J., S. Sarkar, D. A. Widdick, E. A. Espeso, M. Orejas, J. Mungroo, M. A. Penalva, and H. N. Arst, Jr. 1995. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 14:779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Diepen, A., T. van der Straaten, S. M. Holland, R. Janssen, and J. T. van Dissel. 2002. A superoxide-hypersusceptible Salmonella enterica serovar Typhimurium mutant is attenuated but regains virulence in p47phox−/− mice. Infect. Immun. 70:2614-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 22.Vignais, P. V. 2002. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell. Mol. Life Sci. 59:1428-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]