Abstract
Context
Type 1 (T1D) and type 2 diabetes (T2D) are associated with an elevated incidence of infectious diseases and a higher risk of infections-related hospitalization and death.
Objective
In this study, we delineated the “vaccinome” landscape obtained with a large immunization schedule offered by the Regional Government of Lombardy in a cohort of 618 396 patients with diabetes (T1D and T2D).
Methods
Between September 2021 and September 2022, immunization coverage for influenza, meningococcus, pneumococcus, and herpes zoster was obtained from the public computerized registry of the health care system of Lombardy Region (Italy) in 618 396 patients with diabetes and in 9 534 087 subjects without diabetes. Type of diabetes, age, mortality, and hospitalizations were retrospectively analyzed in vaccinated and unvaccinated patients.
Results
Among patients with diabetes (T1D and T2D), 44.6% received the influenza vaccine, 10.9% the pneumococcal vaccine, 2.5% the antimeningococcus vaccine, and 0.7% the antizoster vaccine. Patients with diabetes immunized for influenza, zoster, and meningococcus showed a 2-fold overall reduction in mortality risk and a decrease in hospitalizations. A 3-fold lower risk of mortality and a decrease in hospitalizations for both cardiac and pulmonary causes were also observed after influenza, zoster, and meningococcus immunization in older patients with diabetes.
Conclusion
Immunization coverage is still far from the recommended targets in patients with diabetes. Despite this, influenza vaccination protected nearly 3800 per 100 000 patients with diabetes from risk of death. The overall impressive decrease in mortality and hospitalizations observed in vaccinated patients strengthens the need for scaling up the “vaccinome” landscape in patients with diabetes.
Keywords: diabetes, immunization coverage, vaccines, mortality, hospitalization
Type 1 (T1D) and type 2 diabetes (T2D) are associated with an increased incidence and severity of infectious diseases, which leads to a greater risk for hospitalization and death (1-4). Influenza is the most frequent infectious disease in patients with diabetes (5), with a 4-fold increased risk of death or of being admitted to hospital for any cause (6, 7). Despite being recommended in at-risk patients (8), the protective effect of influenza vaccination in preventing all-cause mortality and complications in patients with diabetes remains unexplored, with data from randomized trials being still unavailable (7, 9). Adult patients with type 1 diabetes (T1D) and type 2 diabetes (T2D) have also an increased risk of death, hospitalization, and respiratory failure due to pneumococcal infection and community-acquired pneumonia (10-12). While the protection offered by pneumococcal vaccination in reducing morbidity and mortality has been demonstrated in the general population (13), very little is known in patients with diabetes. Another frequent infectious disease in patients with diabetes (T1D and T2D) is the herpes zoster infection (14), with an yearly incidence of 7.23 to 9.36/1000. Herpes zoster is associated with a reduced quality of life due to postherpetic neuralgia and ophthalmic complications leading to blindness (15, 16). Unfortunately, data on the protection provided by herpes zoster immunization in patients with diabetes are scanty and incomplete (17). Bacterial meningitis occurs in 3.15 per 100 000 patients with diabetes per year and the risk is 2-fold higher than in subjects without diabetes, with meningococcus accounting for 10% of cases (18). As mortality and the hospitalization rate of bacterial meningitis are higher in patients with diabetes (19), immunization for meningococcus is highly recommended but poorly explored. Indeed, less than 65% of patients with T1D are vaccinated for meningococcus (20) and data from large cohorts are lacking. Based on these premises, in this study we analyzed the full landscape of vaccination coverage, (ie, vaccinome landscape), in nearly 620 000 patients with diabetes (T1D and T2D), and evaluated the protection offered by 4 vaccines (influenza, meningococcus, pneumococcus, and herpes zoster) strongly recommended by the current guidelines of the Lombardy Healthcare System. Our findings will establish a starting point for the immunization coverage in patients with diabetes, allowing it to be implemented and expanded in this high-risk population nationwide and worldwide.
Materials and Methods
In the past 20 years, the Lombardy Region has developed a system for classifying all persons registered within the health care system (database of persons registered with a general practitioner) according to their use of major health care services (hospitalizations, outpatient consultations, pharmaceutical) and whether they are exempt from copayment fees for disease-specific medications and health care services. This retrospective study was conducted on the target population with diabetes in the Lombardy area consisting of 618 396 patients with diabetes (T1D or T2D). Two data sources were used for carrying out the current analysis. The first was the Healthcare Utilization Database, employed in Regional Health Service management, which collects information including hospital discharge records supplied by public or private hospitals (primary diagnosis and performed procedures coded according to the ICD-9-CM classification system), and outpatient dispensation of drugs reimbursed to pharmacies after filing doctors’ prescriptions (coded according to the Anatomical Therapeutic Chemical classification system). The second data source was the vaccination registry, which monitors and evaluates the vaccination campaigns, and which collects information on date, type, and dose of each vaccine that is dispensed. All the different data are interconnected because a single individual identification code is used to recognize each patient by all databases. To preserve privacy, each identification code was deidentified automatically, with this inverse process being allowed only for the Regional Health Service on request from judicial authorities. Informed consent was not obtained since this is a registry-based study based on a retrospective analysis carried on an administrative database (21), where no identifiable information is present. While anti-influenza vaccination is seasonal and patients were included in the analysis as vaccinated within the 2021-2022 campaign, antipneumococcus (conjugate, polysaccharide), antimeningococcus (conjugate), and antizoster (Shingrix, recombinant) vaccinations are administered once, with boosters or annual administration recommended based on the Italian Ministry of Health guidelines (Table S1 (22)). Therefore, vaccinated patients were included based on the registry data but they may have received the vaccination at earlier timepoints. As immunization coverage, patients who did not receive the influenza vaccination during the 2021-2022 campaign were considered not vaccinated. Moreover, patients who had never received antipneumococcus, antimeningococcus, and antizoster were also considered not vaccinated. Patients with newly diagnosed diabetes within the observation period were not included. All the methods adhered to relevant ethical guidelines for handling human data. For statistical analysis a chi-square/Fisher exact test was used to examine the proportion of patients with diabetes receiving or not the anti-influenza vaccination during the 2021-2022 campaign, to examine the proportion of patients having received at least once antizoster, antimeningococcus, and antipneumococcus immunization, and lastly the proportion of patients with diabetes who developed clinical outcomes identified as mortality and admission to hospital. In particular, for each of the 4 immunization strategies evaluated, 3 clinical outcomes were analyzed: mortality (patients with diabetes were considered for whom the date of death is equal to or before September 30, 2022), generic hospitalizations and hospitalizations for cardiac or respiratory diseases (all hospitalization date between January 9, 2021 and September 30, 2022, were considered; the patient was counted if the hospitalization date was in that time window or, for vaccinated, if it was after the vaccine date). Analysis of the crude odds ratio and of the number of patients needed to treat/vaccinate (NNT/NTV) were also included for each outcome to establish the unadjusted risk of developing the above-mentioned outcomes and to evaluate the protection offered by the vaccination strategy administered. NNT was calculated as reciprocal of the attributable risk. All statistical analysis were performed using SAS and R Statistical Software (version 4.2.2) and GraphPad Software LLC (Version 9.5.1).
Results
Study Subjects and Vaccination Coverage
As of September 30, 2022, the population of the Lombardy area in Italy consisted of 10 152 483 people, of whom 618 396 were patients with diabetes (based on the regional exempt coding system) and 9 534 087 were subjects without diabetes. Among the 618 396 patients with diabetes, 15 961 were diagnosed with T1D and 602 435 with T2D. We retrospectively analyzed the immunization coverage for influenza, pneumococcus, meningococcus, and herpes zoster in both patients with and without diabetes as shown in Table 1. First, we observed that 44.6% of patients with diabetes received influenza vaccination compared with 16.0% of subjects without diabetes, leading to a nearly 3-fold increase in influenza vaccination coverage (Table 1). Conversely, we observed a smaller coverage for antipneumococcal vaccine in patients with diabetes compared with the general population (10.9% vs 16.1%, Table 1). With regards to the antimeningococcal vaccine, coverage in the general population was higher than in patients with diabetes (21.2% vs 2.5%) (Table 1). Analysis of immunization coverage for the antizoster vaccine demonstrated that a higher proportion of patients with diabetes received the antizoster vaccine than subjects without diabetes (0.7% vs 0.2%), although coverage remains below 1%.
Table 1.
Summary of the immunization coverage recorded for the population of the Lombardy Region and grouped based on age, type of vaccination (influenza, pneumococcus, meningococcus, and zoster), and presence or absence of diabetes
| Vaccinated | Not vaccinated | |||||||
|---|---|---|---|---|---|---|---|---|
| Influenza | Pneumococcus | Meningococcus | Zoster | Influenza | Pneumococcus | Meningococcus | Zoster | |
| Patients without diabetes | ||||||||
| Age (n) | ||||||||
| 0-17 (1 493 739) | 138 955 (9.3%) | 1 182 116 (79.1%) | 1 316 183 (88.1%) | 8 (<0.1%) | 1 354 784 (90.7%) | 311 623 (20.9%) | 177 556 (11.9%) | 1 493 731 (<99.9%) |
| 18-49 (3 705 428) | 121 118 (3.3%) | 104 846 (2.8%) | 621 426 (16.8%) | 700 (<0.1%) | 3 584 310 (96.7%) | 3 600 582 (97.2%) | 3 084 002 (83.2%) | 3 704 728 (<99.9%) |
| 50-64 (2 255 313) | 189 875 (8.4%) | 39 926 (1.8%) | 62 085 (2.7%) | 2323 (0.1%) | 2 065 438(91.6%) | 2 215 387 (98.2%) | 2 193 228(97.2%) | 2 252 990 (99.9%) |
| ≥65 (2 079 607) | 1 076 461(51.8%) | 212 136 (10.2%) | 19 036 (0.9%) | 18 122 (0.9%) | 1 003 146 (48.2%) | 1 867 471(89.8%) | 2 060 571(99.1%) | 2 061 485 (99.1%) |
| Total (9 534 087) | 1 526 409 (16.0%) | 1 539 024 (16.1%) | 2 018 730 (21.2%) | 21 153 (0.2%) | 8 007 678 (84.0%) | 7 995 063 (83.9%) | 7 515 357 (78,8%) | 9 512 934 (99.8%) |
| Patients with diabetes | ||||||||
| Age (n) | ||||||||
| 0-17 (2198) | 706 (32.1%) | 1612 (73.3%) | 1898 (86.3%) | 0 (0.0%) | 1492 (67.9%) | 586 (26.7%) | 300 (13.6%) | 2198 (100%) |
| 18-49 (74 635) | 7610 (10.2%) | 2425 (3.3%) | 4855 (6.5%) | 49 (<0.1%) | 67 025 (89.8%) | 72 210 (96.7%) | 69 780 (93.5%) | 74 586 (99.9%) |
| 50-64 (126 581) | 30 922 (24.4%) | 7431 (5.9%) | 4517 (3.6%) | 424 (0.3%) | 95 659 (75.6%) | 119 150 (94.1%) | 122 064 (96.4%) | 126 157 (99.7%) |
| ≥65 (414 982) | 236 641 (57.0%) | 55 731 (13.4%) | 4323 (1.0%) | 3782 (0.9%) | 178 341 (43.0%) | 359 251 (86.6%) | 410 659 (99.0%) | 411 200 (99.1%) |
| Total (618 396) | 275 879 (44.6%) | 67 199 (10.9%) | 15 593 (2.5%) | 4255 (0.7%) | 342 517 (55.4%) | 551 197 (89.1%) | 602 803 (97.5%) | 614 141 (99.3%) |
Age (n) 0-17, 18-49, 50-64, ≥ 65: In the left column: total number of patients for each age group. In brackets: percentage of subjects within each age group who received or not the vaccination in the corresponding column, grouped for presence/absence of diabetes. Total: In the left column: total number of patients. In brackets: percentage of subjects out of total who received or did not the vaccination in the corresponding column.
Vaccination coverage by age and type of diabetes
When stratified by age groups, patients with diabetes having received the influenza vaccine were primarily 65 years old or older (85.8% of the vaccinated diabetic population), with 57% of vaccinated patients compared with 43% unvaccinated subjects in this age group (Table 1). This observation was paralleled in patients receiving the pneumococcus vaccine, in whom the majority were aged ≥65 years (82.9% of the vaccinated diabetic population). When considering patients with diabetes having received the antimeningococcal vaccine, the majority were ≥18 years old (88.0% of the vaccinated diabetic population). Vaccination in subjects without diabetes was recorded mainly in the youngest age group (65.2% of the vaccinated subjects without diabetes aged <18 years) (Table 1). As immunization with the antimeningococcal vaccine is strongly recommended in patients with T1D based on the Italian Ministry of Health guidelines (Table S1 (22)), we conducted a subgroup analysis that demonstrated that patients with T1D receive immunization with the antimeningococcal vaccine in a higher proportion than patients with T2D (23.5% vs 2.0%, P < .0001). Finally, the antizoster vaccine was more frequently administered to patients with diabetes aged ≥65 years (88.8% of the vaccinated diabetic population). Overall, patients with diabetes, particularly in the age group greater than 65 years old, demonstrated better immunization coverage than the same age group of subjects without diabetes.
Anti-influenza Vaccine
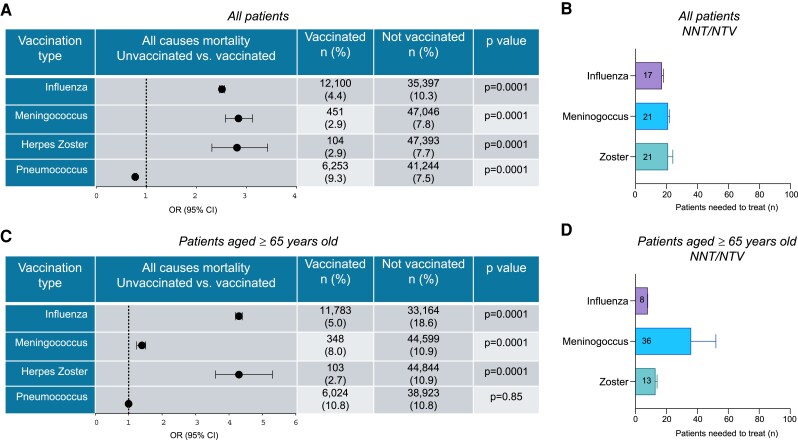
Of the 618 396 patients with diabetes, 275 879 (44.6%) were vaccinated during the influenza seasonal immunization campaign (September 2021-April 2022). We next analyzed the association between immunization coverage and clinical outcomes as follows: mortality rate (determined at September 30, 2022), hospitalization rate, and hospitalization rate for cardiac and/or pulmonary-related causes. Our analysis demonstrated that patients with diabetes and not receiving influenza vaccination experienced an elevated mortality rate (10.3% vs 4.4%, P < .0001), with a 2.5-fold increase in the odds of death compared with vaccinated subjects, (OR 2.5, 95% CI 2.4 to 2.6), and a notable low NNT/NTV to achieve the outcome (Fig. 1A and 1B). The rate of hospitalization, both for generic and cardiac and/or pulmonary-related causes was similar between the 2 groups examined, with 15.8% of unvaccinated patients with diabetes admitted to hospital for all causes compared with 17.2% patients with diabetes receiving the influenza vaccination. Of the hospitalizations for cardiac or respiratory conditions, 3.2% were observed in nonvaccinated compared with 3.6% in vaccinated patients (Table 2).
Figure 1.
Immunization for influenza, meningococcus, and herpes zoster decreases the mortality risk in patients with diabetes. (A) Forest plot showing the mortality risk expressed as odds ratio (OR) in all patients with diabetes included in the study receiving or not the vaccination for influenza, meningococcus, herpes zoster, and pneumococcus. The number (percentage) of patients with the event is presented. (B) Bar graph depicting the number needed to vaccinate/treat (NNT), presented as mean ± 95% CI, to prevent the outcome (death) for the vaccinations analyzed as a surrogate measure of vaccination efficacy in all patients with diabetes. (C) Forest plot showing the mortality risk expressed as odds ratio (OR) in patients with diabetes aged ≥65 years receiving or not the vaccination for influenza, meningococcus, herpes zoster, and pneumococcus. The number (percentage) of patients with the event is presented. (D) Bar graph depicting the number needed to vaccinate/treat (NNT), presented as mean ± 95% CI, to prevent the outcome (death) for the vaccinations analyzed as a surrogate measure of vaccination efficacy in patients with diabetes aged ≥65 years.
Table 2.
Summary of all causes and cardiac/pulmonary-related hospitalization rate in patients with diabetes immunized or not for influenza, pneumococcus, meningococcus, and herpes zoster
| Type of vaccination | Clinical outcomes | |||||
|---|---|---|---|---|---|---|
| Hospitalization any cause | Hospitalization cardiac/pulmonary causes | |||||
| Vaccinated n (%) | Not vaccinated n (%) | P value | Vaccinated n (%) | Not vaccinated n (%) | P value | |
| Influenza | 47 452 (17.2) | 54 194 (15.8) | .0001 | 10 055 (3.6) | 11 012 (3.2) | .0001 |
| Meningococcus | 2331 (14.9) | 99 315 (16.5) | .0001 | 234 (1.5) | 20 833 (3.5) | .0001 |
| Herpes zoster | 750 (17.6) | 100 896 (16.4) | .04 | 137 (3.2) | 20 930 (3.4) | .5 |
| Pneumococcus | 13 233 (19.6) | 88 413 (16.0) | .0001 | 3145 (4.6) | 17 922 (3.2) | .0001 |
In brackets: percentage of subjects with diabetes who were hospitalized for any cause or for cardiac/pulmonary causes having received or not the type of vaccination reported in the corresponding row.
Subgroup analysis by age
In a subgroup analysis, age higher than 65 years was associated with a higher mortality rate in patients with diabetes and not receiving influenza vaccination. Indeed, immunization coverage for influenza reduced the mortality risk by 3.73 times in the elderly population with diabetes compared with unvaccinated patients (5.0% vs 18.6%, P < .0001) with a very small NNT/NTV (Fig. 1C and 1D; Fig. S1A and S1B (22)). With regards to hospitalization rate for all causes, there is a decreased percentage of patients with diabetes immunized for influenza who were admitted to hospital compared with patients with diabetes who did not receive the vaccination (18.0% vs 19.4%, P < .0001). This observation was furthermore paralleled when considering the cardiac/pulmonary-related hospitalization rate (4.0% vs 5.1%, P < .0001, OR 1.27 for unvaccinated patients; 95% CI 1.23 to 1.30) (Table 3; Table S2 (22)).
Table 3.
Summary of the general and cardiac/pulmonary-related hospitalization rate in patients with diabetes aged 65 years or older and immunized or not for influenza, pneumococcus, meningococcus, and herpes zoster grouped for age according to the guidelines recommendation
| Type of vaccination | Clinical outcomes in patients aged ≥65 years | |||||
|---|---|---|---|---|---|---|
| Hospitalization any cause | Hospitalization cardiac/pulmonary causes | |||||
| Vaccinated n (%) | Not vaccinated n (%) | P value | Vaccinated n (%) | Not vaccinated n (%) | P value | |
| Influenza | 42 646 (18.0) | 34 590 (19.4) | .0001 | 9548 (4.0) | 9024 (5.1) | .0001 |
| Meningococcus | 839 (19.4) | 76 397 (18.6) | .1 | 146 (3.3) | 18 426 (4.5) | .0003 |
| Herpes zoster | 677 (17.9) | 76 559 (18.6) | .2 | 129 (3.4) | 18 443 (4.5) | .01 |
| Pneumococcus | 11 407 (20.4) | 65 829 (18.3) | .0001 | 2958 (5.3) | 15 614 (4.3) | .0001 |
In brackets: percentage of subjects with diabetes and aged ≥65 years who were hospitalized for any cause or for cardiac/pulmonary causes having received or not the type of vaccination reported in the corresponding row.
Subgroup analysis by type of diabetes
By grouping the population based on the type of diabetes, the mortality rate was statistically significantly different in patients with T2D (5.0% vs 19.0%, P < .0001) and T1D (8.0% vs 23.0%, P < .0001) aged ≥65 years and immunized with the influenza vaccine compared with unvaccinated patients. Our analysis indeed demonstrated a 3 to 4 times lower risk of death in patients with T1D (OR 3.1 for unvaccinated patients, 95% CI 2.1 to 4.0) or T2D (OR 4.3 for unvaccinated patients, 95% CI 4.2 to 4.4) aged ≥65 years and receiving the influenza vaccine compared with unvaccinated patients. The rate of all-causes hospitalization was also reduced in this subgroup of patients, particularly in those with T1D, but to a lesser extent (28.7% vs 40.0%, P = .0006).
Antimeningococcus Vaccine
In our retrospective analysis, 15 593 (2.5%) patients with diabetes received immunization for meningococcus. We observed that the rate of mortality in subjects receiving this type of vaccine was lower than in unvaccinated subjects (2.9% vs 7.8%, P < .0001) and the risk of death was significantly reduced (OR 2.8 for unvaccinated patients, 95% CI 2.6 to 3.1), and further paralleled by a low NNT/NTV (Fig. 1A and 1B). The rate of hospitalization for all causes and for cardiac/pulmonary causes was also reduced in the vaccinated population (Table 2). Patients with diabetes admitted to hospital for all causes were 14.9% in the vaccinated group and 16.5% in the nonvaccinated group (P < .0001, Table 2). Cardiac and/or pulmonary-related hospitalizations occurred in 1.5% of vaccinated subjects compared with 3.5% occurrences in subjects not vaccinated (P < .0001, Table 2).
Subgroup analysis by age
When grouped by age, patients aged ≥65 years undergoing immunization showed a lower mortality risk and a small NNT/NTV (Fig. 1C and 1D; Fig. S1A and S1B (22)). A decrease in admission to hospital was also observed in the subgroup of patients aged ≥65 years, particularly in hospitalizations for cardiac/pulmonary causes more so than in hospitalizations registered for all causes (Table 3; Table S2 (22)).
Subgroup analysis by type of diabetes
When considering patients with T2D, the mortality rate was lower in vaccinated than in unvaccinated subjects (8.0% vs 10.8%, P < .0001). This was also confirmed in the younger age groups of patients with T1D, with a 0.1% mortality in vaccinated compared with 0.6% in unvaccinated patients (P < .0001).
Antizoster Vaccine
Zoster vaccinated subjects represented a small group in our retrospective study, with only 0.7% of patients with diabetes having adhered to this vaccination campaign. Despite the small number of patients included in the analysis, the comparison between the vaccinated and nonvaccinated population showed that mortality rate was lower in vaccinated patients (2.9% vs 7.7%, P < .0001). This led to a significant reduced risk of mortality in immunized patients (OR 2.8 for unvaccinated patients, 95% CI 2.3 to 3.4) and a relatively low NNT/NTV to achieve the outcome (Fig. 1A and 1B). Further analysis demonstrated similar hospitalization rates both for all causes and for cardiac/pulmonary diseases in patients with diabetes receiving or not receiving the vaccination (17.6% vs 16.4%, P = .04; 3.2% vs 3.4%, P = .5) (Table 2).
Subgroup analysis by age
Among subjects aged ≥65 years, in whom this vaccination is recommended, the decrease in mortality rate was particularly evident in vaccinated vs nonvaccinated patients with diabetes (2.7% vs 10.9%, P < .0001), with a low NNT/NTV (Fig. 1C and 1D; Fig. S1A and S1B (22)). A higher risk of death in the absence of antizoster immunization was evident (OR 4.3 for unvaccinated patients, 95% CI 3.6 to 5.3). No decrease in the rate of hospitalizations for all causes in patients aged above or below 65 years and receiving the vaccine was observed. Nonetheless, a reduction in the proportion of vaccinated patients with diabetes admitted to hospital for cardiac/pulmonary diseases was noted (Table 3; Table S2 (22)).
Subgroup analysis by type of diabetes
A reduction in mortality rate was confirmed in vaccinated patients with T2D (2.4% vs 7.9%, P < .0001), with a 3-fold increase in the odds of death for unvaccinated patients (OR 3.4 for unvaccinated patients, 95% CI 2.8 to 4.1). This was not observed in patients with T1D (1.7% vs 1.3%, P = .5).
Antipneumococcal Vaccine
Of the 618 396 patients with diabetes included in our retrospective analysis, 67 199 (10.9%) were immunized with the antipneumococcal vaccine. The analysis of mortality (9.3% vs 7.5%), hospitalization for all causes (19.6% vs 16.0%) and cardiopulmonary-related causes (4.6 vs 3.2%) in patients with diabetes showed no improvement in vaccinated compared with unvaccinated subjects (Fig. 1A and Table 2). No decrease related to the antipneumococcal vaccination was observed with regards to the hospitalization rate, which was slightly increased in vaccinated subjects. As far as hospitalizations are concerned, 19.6% of vaccinated patients with diabetes were admitted to hospital for all causes compared with 16.0% of those who were not vaccinated (Table 2). These data were paralleled in the analysis of cardiac/pulmonary hospitalizations, with 4.6% of patients with diabetes hospitalized and having received immunization compared with 3.2% of unvaccinated subjects (Table 2).
Subgroup analysis by age
When grouped by age, no decrease in mortality rate was observed in the subgroup of patients with diabetes aged 65 and older receiving the vaccine, with respect to nonvaccinated patients (Fig. 1C; Fig. S1A (22)). This observation was also confirmed in the analysis of hospitalizations for all causes: there was no decrease in admissions to hospital registered in vaccinated patients, even when considering cardiac/pulmonary hospitalization rate (5.3% vs 4.3%, Table 3; Table S2 (22)).
Subgroup analysis by type of diabetes
When grouped by type of diabetes, the mortality rate was lower in patients with T1D who received the antipneumococcal vaccination than in unvaccinated patients (0.9% vs 1.4%). No decrease was observed in the vaccinated group of patients with T2D (8.9% vs 7.1%), as noted in the analysis of all patients.
Discussion
In this study on immunization coverage in the Lombardy Region, Italy, we delineated the “vaccinome” landscape in at-risk patients with diabetes, with a particular focus on influenza, meningococcus, pneumococcus, and herpes zoster immunization strategies. These vaccinations are highly recommended by the Italian Healthcare System (Table S1 (22)) and by the scientific community worldwide in at-risk patients (23), such as those with diabetes. Our study confirmed that the proportion of patients immunized is lower than that foreseen by the Italian Ministry of Health (24). This is extremely relevant for patients with diabetes, as our data demonstrated that under-vaccination was associated with a 2- to 3-fold increased risk of mortality for at least 3 out of the 4 vaccines examined (influenza, meningococcus, and herpes zoster). Our data further demonstrated that in patients aged ≥65 years, this risk is more evident and it is also accompanied by an increased rate of admission to hospital for cardiac and/or pulmonary diseases. The Italian Ministry of Health set guidelines for influenza vaccination coverage in patients with diabetes, considered subjects at risk, at 75% and 95%, considered the minimum and optimal minimum, respectively (24). In our study, compliance with influenza vaccination was higher in patients with diabetes aged ≥65 years than in subjects without diabetes, with percentages approaching the coverage recommended by the Italian Ministry of Health (Table S1 (22)). However, recommendation for influenza vaccination in patients with diabetes starts from 6 months of age (8), and in our study the proportion of patients with diabetes vaccinated for influenza aged below 18 years was very low, nearly 32%. This suggests that coverage in younger patients is still below the target. With regards to the antipneumococcal vaccination, which is recommended as once in a lifetime in at-risk patients (8, 23) (Table S1 (22)), the target has been set at 75% vaccination coverage (25). In our study, adherence to antipneumococcal immunization was still below the target, with nearly 11% of patients receiving the vaccine. Compliance was higher in patients with T1D, in those aged <18 years, and in patients with T2D aged ≥65 years. The immunization coverage target was not achieved for the antiherpes zoster vaccination (50% coverage set by the national guidelines) (Table S1 (22)), as already observed in other countries (26). Indeed, the low rate of immunization is associated with unawareness of the severity of the disease, lack of immunization campaigns, and poor trust in the efficacy and safety of the vaccine (27). Immunization with meningococcal vaccine of any type is recommended primarily in patients with T1D (28). In our study, we showed that nearly 25% of patients with T1D received this vaccination, mainly aged <18 years and received it to a similar extent as in subjects without diabetes. However, proportions of vaccinated patients grouped by age should be considered with caution, as the age recommendation for each type of vaccination varies and some vaccinations require a booster dose later on. With regards to clinical outcomes, it has been already established that in patients with diabetes under-vaccination is linked to an increased risk of mortality and admission to hospital (21, 29), while the majority of vaccines are safe (30-33). Our study indeed demonstrated that these concepts apply to influenza immunization, particularly for mortality rate, but also to vaccination with antimeningococcus and herpes zoster. This highlights the relevance of achieving the immunization coverage target for each vaccine strategy in patients with diabetes. This is particularly relevant for the herpes zoster vaccination, where data are scant and the disease's morbidity and mortality rate are high (34). The fact that in our study protection from hospitalization was mainly restricted to patients aged ≥65 years strengthens the importance of having full immunization coverage in patients with diabetes, as older patients with diabetes may benefit the most for protection from cardiovascular and/or respiratory diseases. Furthermore, while influenza immunization is offered annually to the general population and is related to a seasonal virus, protection against pneumococcus and meningococcus is offered once in a lifetime. However, vaccination is recommended for patients aged ≥65 years, particularly if the vaccine status is unknown and regardless of the presence of diabetes (8), thus resulting in a more heterogeneous protective effect (25). Also, both bacterial meningitis and pneumonia might be sustained by other microorganisms (eg, Listeria, Klebsiella), which accounts for a reasonable number of hospitalizations and deaths (18, 19). In this regard, the low protective effect observed with the antipneumococcus vaccination may be due to the heterogeneity of pulmonary diseases and on the relative low rate of vaccination observed in the older age groups, which may be more prone to develop pneumonia if suffering from diabetes. Our study is not devoid of limitations. First, it is a retrospective registry-based study, therefore some clinical data are not available. The risk assessment has been based on the mortality rate and unadjusted odds ratio, without performing a multiple comparisons test. Second, the analysis conducted suffered from the small number of patients receiving some vaccinations and the relatively small number of admissions to hospital recorded in some subgroups of patients, which did not allow us to expand our conclusions. Indeed, we acknowledge that low vaccination rates (except for influenza) and low mortality rates in the 1-year analysis may imply a limit to the numbers, thus reducing the power to detect differences. Third, the limited observation period may not allow us to draw long-term projections, particularly for the influenza vaccination, which is seasonal and virus related. However, the width of the epidemic curve and the intensity of 2021-2022 influenza activity were lower than the historical data (35). Moreover, for those vaccines administered once in a lifetime such as antimeningococcus and antipneumococcus, longer studies are needed to better clarify the overall benefits on clinical outcomes. Furthermore, we may consider that different vaccines exist for some infectious agents (eg, herpes zoster, pneumococcus), which may account for differences in effectiveness, and were not explored in the present study. Finally, we may have underestimated the number of patients with diabetes living in the Lombardy area, as those with milder forms of the disease or with other forms of diabetes (monogenic diabetes, secondary diabetes) might not have been included in the regional exempt coding system. However, our study has also some major strengths, such as the high number of patients with diabetes included, nearly 620 000. Another strength lies in the rigor and precision of the data collection handled by the Lombardy Regional Healthcare System. Finally, depicting the “vaccinome” landscape in patients with diabetes (T1D and T2D), including the vaccinations recommended the most by the Italian Ministry of Health in this specific at risk population, is a novelty in this study. In summary, our study delineates immunization coverage in the diabetic population of the Lombardy Region over a year (2021-2022) and demonstrates that, although a relatively high adherence has been recorded, the target set by the Italian Ministry of Health is still far from being achieved. As under-vaccination in our study was associated with great mortality, it is mandatory to sensitize the public and the health care community to the clinical advantages that vaccinations may offer to patients at risk. The influenza immunization campaign reported in this study is the prime example of the benefits of vaccination, which secured protection from risk of death in nearly 25 000 among 620 000 patients with diabetes.
Acknowledgments
We thank the “Fondazione Romeo e Enrica Invernizzi” for extraordinary support.
Abbreviations
- NNT/NTV
number of patients needed to treat/vaccinate
- T1D
type 1 diabetes
- T2D
type 2 diabetes
Contributor Information
Francesca D’Addio, Division of Endocrinology, ASST Fatebenefratelli-Sacco, 20157 Milan, Italy; International Center for T1D, Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, Department of Biomedical and Clinical Sciences, Università Degli Studi di Milano, 20157 Milan, Italy.
Elisa Lazzaroni, Division of Endocrinology, ASST Fatebenefratelli-Sacco, 20157 Milan, Italy; International Center for T1D, Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, Department of Biomedical and Clinical Sciences, Università Degli Studi di Milano, 20157 Milan, Italy.
Maria Elena Lunati, Division of Endocrinology, ASST Fatebenefratelli-Sacco, 20157 Milan, Italy.
Giuseppe Preziosi, ARIA S.p.A. (The Innovation and Procurement Regional Company of Regione Lombardia), 20124 Milano, Lombardia, Italy.
Michele Ercolanoni, ARIA S.p.A. (The Innovation and Procurement Regional Company of Regione Lombardia), 20124 Milano, Lombardia, Italy.
Giulio Turola, ARIA S.p.A. (The Innovation and Procurement Regional Company of Regione Lombardia), 20124 Milano, Lombardia, Italy.
Chiara Marrocu, Postgraduate School in Public Health, Department of Biomedical Sciences for Health, University of Milan, 20133 Milan, Italy.
Giovanni Cicconi, Postgraduate School in Public Health, Department of Biomedical Sciences for Health, University of Milan, 20133 Milan, Italy.
Sudwaric Sharma, Postgraduate School in Public Health, Department of Biomedical Sciences for Health, University of Milan, 20133 Milan, Italy.
Simona Scarioni, Postgraduate School in Public Health, Department of Biomedical Sciences for Health, University of Milan, 20133 Milan, Italy.
Laura Montefusco, Division of Endocrinology, ASST Fatebenefratelli-Sacco, 20157 Milan, Italy.
Ida Pastore, Division of Endocrinology, ASST Fatebenefratelli-Sacco, 20157 Milan, Italy; International Center for T1D, Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, Department of Biomedical and Clinical Sciences, Università Degli Studi di Milano, 20157 Milan, Italy.
Paola Silvia Morpurgo, Division of Endocrinology, ASST Fatebenefratelli-Sacco, 20157 Milan, Italy.
Antonio Rossi, International Center for T1D, Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, Department of Biomedical and Clinical Sciences, Università Degli Studi di Milano, 20157 Milan, Italy; IRCCS Ospedale Galeazzi—Sant’Ambrogio, Internal Medicine, 20157 Milan, Italy.
Alessandra Gandolfi, Division of Endocrinology, ASST Fatebenefratelli-Sacco, 20157 Milan, Italy.
Camilla Tinari, Division of Endocrinology, ASST Fatebenefratelli-Sacco, 20157 Milan, Italy.
Giada Rossi, Division of Endocrinology, ASST Fatebenefratelli-Sacco, 20157 Milan, Italy; International Center for T1D, Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, Department of Biomedical and Clinical Sciences, Università Degli Studi di Milano, 20157 Milan, Italy.
Moufida Ben Nasr, International Center for T1D, Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, Department of Biomedical and Clinical Sciences, Università Degli Studi di Milano, 20157 Milan, Italy.
Cristian Loretelli, International Center for T1D, Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, Department of Biomedical and Clinical Sciences, Università Degli Studi di Milano, 20157 Milan, Italy.
Roberta Maria Fiorina, International Center for T1D, Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, Department of Biomedical and Clinical Sciences, Università Degli Studi di Milano, 20157 Milan, Italy.
Baldassarre Grassa, U.O.S. Diabetologia ASST Lariana, P.O. Mariano Comense, CO 22066, Italy.
Rosa Terranova, Division of Diabetology, Niguarda Hospital, 20162 Milan, Italy.
Loredana Bucciarelli, International Center for T1D, Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, Department of Biomedical and Clinical Sciences, Università Degli Studi di Milano, 20157 Milan, Italy; IRCCS MultiMedica Sesto San Giovanni, 20099 Milano, Italy.
Cesare Berra, IRCCS MultiMedica Sesto San Giovanni, 20099 Milano, Italy.
Danilo Cereda, Directorate General for Health, 20124 Milano, Lombardia, Italy.
Gianvincenzo Zuccotti, Buzzi Children's Hospital, Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, Department of Biomedical and Clinical Sciences, Università Degli Studi di Milano, 20157 Milan, Italy.
Catia Rosanna Borriello, Directorate General for Health, 20124 Milano, Lombardia, Italy.
Paolo Fiorina, Division of Endocrinology, ASST Fatebenefratelli-Sacco, 20157 Milan, Italy; International Center for T1D, Pediatric Clinical Research Center Romeo ed Enrica Invernizzi, Department of Biomedical and Clinical Sciences, Università Degli Studi di Milano, 20157 Milan, Italy; Nephrology Division, Boston Children's Hospital, Harvard Medical School, Boston, MA 02115, USA.
Funding
F.D’A. is supported by Ministero della Salute grant RF-2021-12372897 and by the Ministero dell’Universita e della Ricerca grant 2022JY9CP. P.F., C.L., and M.BN. are supported by the Ministero dell’Universita e della Ricerca grant 20229ZA2YF, 2022NH9MXB, and 20225HWJMB, respectively.
Author Contributions
F.D’A., E.L., and ME.L. designed the study, analyzed data, and wrote the paper; G.P., M.E., G.T., C.M., G.C., L.M., I.P., PS.M., A.R., S.S, S.S., A.G., C.T., G.R., M.BN., C.L., and RM.F. collected and analyzed data; R.T., B.G., L.B., C.B., D.C., CR.B., and G.Z. edited the paper; P.F. conceived the idea, designed the study, and wrote and edited the paper. P.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
The authors have nothing to disclose.
Data Availability
The data that support the findings of this study are available from Regione Lombardia Health Service, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from Regione Lombardia, Directorate General for Health, upon reasonable request.
References
- 1. Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26(2):510‐513. [DOI] [PubMed] [Google Scholar]
- 2. Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018;41(3):513‐521. [DOI] [PubMed] [Google Scholar]
- 3. Magliano DJ, Harding JL, Cohen K, Huxley RR, Davis WA, Shaw JE. Excess risk of dying from infectious causes in those with type 1 and type 2 diabetes. Diabetes Care. 2015;38(7):1274‐1280. [DOI] [PubMed] [Google Scholar]
- 4. Solerte SB, D’Addio F, Trevisan R, et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care. 2020;43(12):2999‐3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goeijenbier M, van Sloten TT, Slobbe L, et al. Benefits of flu vaccination for persons with diabetes mellitus: a review. Vaccine. 2017;35(38):5095‐5101. [DOI] [PubMed] [Google Scholar]
- 6. Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347(aug23 1):f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dicembrini I, Silverii GA, Clerico A, et al. Influenza: diabetes as a risk factor for severe related-outcomes and the effectiveness of vaccination in diabetic population. A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2023;33(6):1099‐1110. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes A . 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1)(Supplement_1):S34‐S45. [DOI] [PubMed] [Google Scholar]
- 9. Vamos EP, Pape UJ, Curcin V, et al. Effectiveness of the influenza vaccine in preventing admission to hospital and death in people with type 2 diabetes. CMAJ. 2016;188(14):E342‐EE51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muller LM, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41(3):281‐288. [DOI] [PubMed] [Google Scholar]
- 11. Kornum JB, Thomsen RW, Riis A, Lervang HH, Schonheyder HC, Sorensen HT. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care. 2008;31(8):1541‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yende S, van der Poll T, Lee M, et al. The influence of pre-existing diabetes mellitus on the host immune response and outcome of pneumonia: analysis of two multicentre cohort studies. Thorax. 2010;65(10):870‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fisman DN, Abrutyn E, Spaude KA, Kim A, Kirchner C, Daley J. Prior pneumococcal vaccination is associated with reduced death, complications, and length of stay among hospitalized adults with community-acquired pneumonia. Clin Infect Dis. 2006;42(8):1093‐1101. [DOI] [PubMed] [Google Scholar]
- 14. Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of Herpes Zoster and its complications. J Infect. 2013;67(5):463‐469. [DOI] [PubMed] [Google Scholar]
- 15. Suaya JA, Chen SY, Li Q, Burstin SJ, Levin MJ. Incidence of herpes zoster and persistent post-zoster pain in adults with or without diabetes in the United States. Open Forum Infect Dis. 2014;1(2):ofu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang CT, Lee CY, Sung HY, Liu SJ, Liang PC, Tsai MC. Association between diabetes mellitus and the risk of herpes zoster: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2022;107(2):586‐597. [DOI] [PubMed] [Google Scholar]
- 17. Silverii GA, Clerico A, Fornengo R, et al. Efficacy and effectiveness of Herpes zoster vaccination in adults with diabetes mellitus: a systematic review and meta-analysis of clinical trials and observational studies. Acta Diabetol. 2023;60(10):1343‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Veen KE, Brouwer MC, van der Ende A, van de Beek D. Bacterial meningitis in diabetes patients: a population-based prospective study. Sci Rep. 2016;6(1):36996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pomar V, de Benito N, Mauri A, Coll P, Gurgui M, Domingo P. Characteristics and outcome of spontaneous bacterial meningitis in patients with diabetes mellitus. BMC Infect Dis. 2020;20(1):292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giatraki V, Dimitriou H, Pappas A, et al. Vaccine coverage in children, adolescents and adults with type 1 diabetes and their close contacts in Crete. Hum Vaccin Immunother. 2021;17(11):4291‐4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Modin D, Claggett B, Kober L, et al. Influenza vaccination is associated with reduced cardiovascular mortality in adults with diabetes: a nationwide cohort study. Diabetes Care. 2020;43(9):2226‐2233. [DOI] [PubMed] [Google Scholar]
- 22. D’Addio F. Supplementary Information for “Vaccinome landscape in nearly 620,000 patients with diabetes”. 2024. 10.13130/RD_UNIMI/VAN8NJ [DOI]
- 23. Andreoni M, Sticchi L, Nozza S, et al. Recommendations of the Italian society for infectious and tropical diseases (SIMIT) for adult vaccinations. Hum Vaccin Immunother. 2021;17(11):4265‐4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Health IMo . Accessed December 1, 2023. https://www.salute.gov.it/portale/influenza/dettaglioContenutiInfluenza.jsp?lingua=italiano&id=679&area=influenza&menu=vuoto
- 25. Del Riccio M, Boccalini S, Cosma C, et al. Effectiveness of pneumococcal vaccination on hospitalization and death in the adult and older adult diabetic population: a systematic review. Expert Rev Vaccines. 2023;22(1):1179‐1184. [DOI] [PubMed] [Google Scholar]
- 26. Papagianni M, Metallidis S, Tziomalos K. Herpes zoster and diabetes mellitus: a review. Diabetes Ther. 2018;9(2):545‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Q, Yang L, Li L, Liu C, Jin H, Lin L. Willingness to vaccinate against herpes zoster and its associated factors across WHO regions: global systematic review and meta-analysis. JMIR Public Health Surveill. 2023;9:e43893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prevention . CfDCa. Recommended Vaccines for Adults with Health Conditions. Accessed December 1, 2023. https://www.cdc.gov/meningococcal/vaccines/index.html
- 29. Lin CS, Chang CC, Yeh CC, Chang YC, Chen TL, Liao CC. Outcomes following diabetes admission in patients who had influenza vaccination: a population-based cohort study. Diabetes Res Clin Pract. 2022;189:109930. [DOI] [PubMed] [Google Scholar]
- 30. D’Addio F, Sabiu G, Usuelli V, et al. Immunogenicity and safety of SARS-CoV-2 mRNA vaccines in a cohort of patients with type 1 diabetes. Diabetes. 2022;71(8):1800‐1806. [DOI] [PubMed] [Google Scholar]
- 31. Dos Santos G, Tahrat H, Bekkat-Berkani R. Immunogenicity, safety, and effectiveness of seasonal influenza vaccination in patients with diabetes mellitus: a systematic review. Hum Vaccin Immunother. 2018;14(8):1853‐1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gidengil C, Goetz MB, Newberry S, et al. Safety of vaccines used for routine immunization in the United States: an updated systematic review and meta-analysis. Vaccine. 2021;39(28):3696‐3716. [DOI] [PubMed] [Google Scholar]
- 33. Ben Nasr M, D’Addio F, Montefusco L, et al. Indirect and direct effects of SARS-CoV-2 on human pancreatic islets. Diabetes. 2022;71(7):1579‐1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patil A, Goldust M, Wollina U. Herpes zoster: a review of clinical manifestations and management. Viruses. 2022;14(2):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Galli C, Pellegrinelli L, Giardina F, et al. On the lookout for influenza viruses in Italy during the 2021-2022 season: along came A(H3N2) viruses with a new phylogenetic makeup of their hemagglutinin. Virus Res. 2023;324:199033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Regione Lombardia Health Service, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from Regione Lombardia, Directorate General for Health, upon reasonable request.