Fig. 2. A p53 null mutation restores G1/S progression and the activity of Cdk2 in Tsg101-deficient cells.
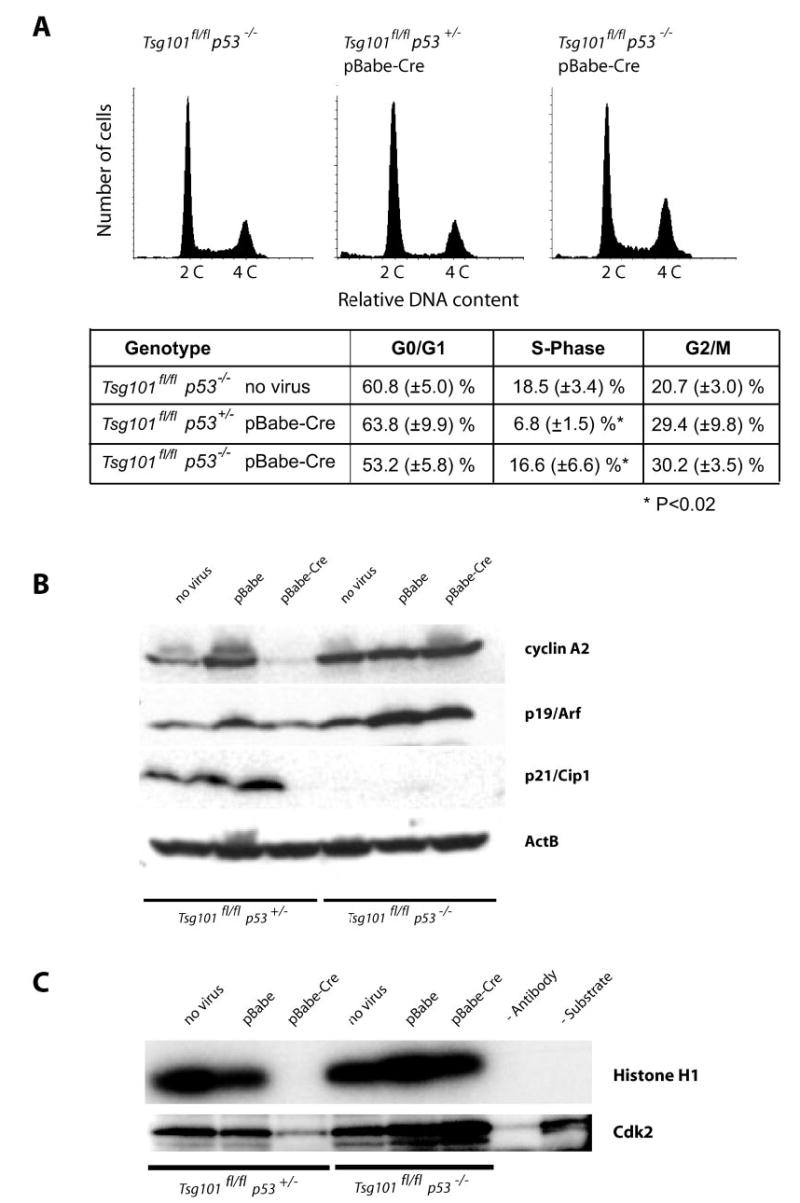
A, flow cytometric analysis of the DNA content of viable Tsg101-deficient MEFs (Tsg101fl/fl, pBabe-Cre) that lack one or two alleles of the p53 gene (p53+/− and p53−/ −) and their uninfected controls (Tsg101fl/fl p53−/ − ). The sub-G1 population of apoptotic cells was gated out in this assay. Note that the relative number of cells in S phase is reduced only in p53 heterozygous knock-out cells lacking Tsg101 but not in Tsg101-deficient MEFs carrying two mutant p53 alleles. B, Western blot analysis of cyclin A (S-phase cyclin) and regulators of G1/S progression (p19Arf and p21Cip) in Tsg101-deficient MEFs that lack one or two copies of p53 and their uninfected controls. Note that cyclin A2 was markedly down-regulated in Tsg101-deficient MEFs that carry at least one functional allele of p53. Immortal p53/p21Cip1-deficient MEFs express high levels of cyclin A2 and p19Arf regardless of the Tsg101 mutation status. C, Cdk2 activity assay using histone H1 as a substrate for phosphorylation. Only Tsg101 knock-out MEFs that carry at least one functional p53 allele exhibit a reduced activity of Cdk2. The complete deletion of p53 and, subsequently, the absence of the Cdk2 inhibitor p21Cip1 restore a normal activity of Cdk2 in Tsg101-deficient cells.
