Fig. 5.
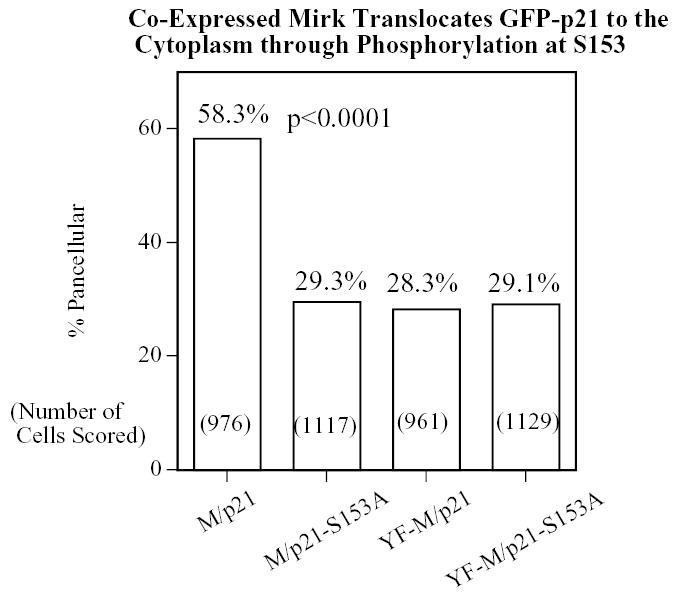
Overexpression of Mirk causes a portion of the wild-type p21 population to translocate to the cytoplasm in a Mirk kinase-dependent manner, but cannot translocate p21 mutated at the Mirk phosphorylation site. NIH3T3 fibroblasts were plated overnight in Labtek 2-well chamber slides (1.5 x 105 cells per well) and then transfected (2 ug plasmid DNA and 4 ul LipofectAMINE 2000 per well) with GFP-p21(p21) or the non-phosphorylatable GFP-p21(p21-S153A), together with either wild-type (M) or kinase inactive (YF-M) Mirk. Cells were transfected in DMEM containing 10% bovine calf serum. After 24 hours of expression, cells were fixed with 4% paraformaldehyde and the GFP-p21 protein was labeled by a 30 minute incubation with mouse anti-GFP monoclonal antibody (Santa Cruz 8334; 1:500), followed by anti-mouse Alexa Fluor 488. Mirk was visualized using rabbit polyclonal antibody to the Mirk C-terminus at 1:500, followed by anti-rabbit Alexa Fluor 594. An average of 1050 GFP-p21 expressing cells were observed for each transfectant. Cells were scored in 8–10 random fields in each of 3 separate preparations and the number of cells labeled for both GFP-p21 and transfected Mirk was determined using a Green/Orange V2 filter set (Chroma) that allowed simultaneous visualization of both fluorophores. Combined counts were analyzed by the Chi square test (Minitab) to determine the significance of differences between conditions. The total number of cells scored per condition is labeled on each bar.
