TABLE 3.
Model parameters: TCA cycle
| Symbol | Value | Units | Description | Eq. | Ref.* |
|---|---|---|---|---|---|
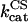 |
3.2 | s−1 | Catalytic constant of CS | 13 | a |
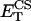 |
0.4 | mM | Concentration of CS | 13 | b |
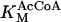 |
1.26 10−2 | mM | Michaelis constant for AcCoA | 13 | a |
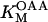 |
6.4 10−4 | mM | Michaelis constant for OAA | 13 | a |
| CKint | 1.0 | mM | Sum of TCA cycle intermediates' concentration | 14 | c |
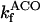 |
12.5 | s−1 | Forward rate constant of ACO | 15 | a |
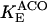 |
2.22 | Equilibrium constant of ACO | 15 | a | |
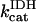 |
1.94–16.3 | s−1 | Rate constant of IDH | 16 | a |
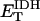 |
0.109 | mM | Concentration of IDH | 16 | b |
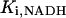 |
0.19 | mM | Inhibition constant by NADH | 16 | a |
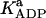 |
6.2 10−2 | mM | Activation constant by ADP | 16 | a |
| [H+] | 2.5 10−5 | mM | Matrix proton concentration | 16 | k |
| kh,1 | 8.1 10−5 | mM | Ionization constant of IDH | 16 | a |
| kh,2 | 5.98 10−5 | mM | Ionization constant of IDH | 16 | a |
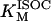 |
1.52 | mM | Michaelis constant for isocitrate | 16 | a |
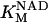 |
0.923 | mM | Michaelis constant for NAD+ | 16 | a |
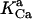 |
0.00141 | mM | Activation constant for Ca2+ | 16 | a |
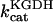 |
0.15–5.0 | s−1 | Rate constant of KGDH | 17 | a |
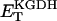 |
0.5 | mM | Concentration of KGDH | 17 | f |
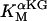 |
1.94 | mM | Michaelis constant for αKG | 17 | a |
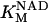 |
38.7 | mM | Michaelis constant for NAD | 17 | a |
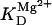 |
0.0308 | mM | Activation constant for Mg2+ | 17 | a |
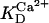 |
1.27 10−3 | mM | Activation constant for Ca2+ | 17 | a |
| nαKG | 1.2 | Hill coefficient of KGDH for αKG | 17 | f | |
| Mg2+ | 0.4 | mM | Mg2+ concentration in mitochondria | 17 | d |
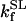 |
0.127 | mM−1 s−1 | Forward rate constant of SL | 18 | f |
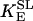 |
3.115 | Equilibrium constant of the SL reaction | 18 | a | |
| [CoA] | 0.02 | mM | Coenzyme A concentration | 18 | b |
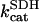 |
1.0 | s−1 | Rate constant of SDH | 19 | f |
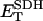 |
0.5 | mM | SDH enzyme concentration | 19 | f |
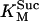 |
3.0 10−2 | mM | Michaelis constant for succinate | 19 | e |
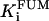 |
1.3 | mM | Inhibition constant by fumarate | 19 | e |
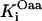 |
0.15 | mM | Inhibition constant by oxalacetate | 19 | e |
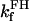 |
0.83 | s−1 | Forward rate constant for FH | 20 | a |
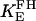 |
1.0 | Equilibrium constant of FH | 20 | a | |
| kh1 | 1.1310−5 | mM | Ionization constant of MDH | 22 | j |
| kh2 | 26.7 | mM | Ionization constant of MDH | 22 | a |
| kh3 | 6.68 10−9 | mM | Ionization constant of MDH | 23 | j |
| kh4 | 5.62 10−6 | mM | Ionization constant of MDH | 23 | j |
| koffset | 3.99 10−2 | pH-independent term in the pH activation factor of MDH | 22 | a | |
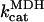 |
2.775 101 | s−1 | Rate constant of MDH | 21 | f |
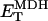 |
0.154 | mM | Total MDH enzyme concentration | 21 | b |
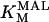 |
1.493 | mM | Michaelis constant for malate | 21 | a |
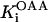 |
3.1 10−3 | mM | Inhibition constant for oxalacetate | 21 | a |
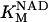 |
0.2244 | mM | Michaelis constant for NAD+ | 21 | a |
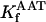 |
0.644 | s−1 | Forward rate constant of AAT | 24 | a |
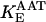 |
6.6 | Equilibrium constant of AAT | 24 | a | |
| KC_ASP | 0.01 | s−1 | First order rate constant of aspartate consumption | 25 | f |
| Model parameters: oxidative phosphorylation | |||||
| ra | 6.394 10−10 | s−1 | Sum of products of rate constants | 26,27 | g,h |
| rb | 1.762 10−13 | s−1 | Sum of products of rate constants | 27 | g,h |
| rc1 | 2.656 10−19 | s−1 | Sum of products of rate constants | 26 | g,h |
| rc2 | 8.632 10−27 | s−1 | Sum of products of rate constants | 26 | g,h |
| r1 | 2.077 10−18 | Sum of products of rate constants | 26,27 | g,h | |
| r2 | 1.728 10−9 | Sum of products of rate constants | 26,27 | g,h | |
| r3 | 1.059 10−26 | Sum of products of rate constants | 26,27 | g,h | |
| ρres | 0.0006–0.05 | mM | Concentration of electron carriers (respiratory complexes I-III-IV) | 26,27 | g,h |
| Kres | 1.35 1018 | Equilibrium constant of respiration | 28 | g,h | |
| ρres(F) | 0.0045 | mM | Concentration of electron carriers (respiratory complexes II-III-IV) | ||
| ΔΨB | 0.05 | V | Phase boundary potential | 26,27,31 | g,h |
| g | 0.85 | Correction factor for voltage | 26,27 | g,h | |
| Kres(F) | 5.765 1013 | Equilibrium constant of FADH2 oxidation | 29 | ||
| [FADH2] | 1.24 | mM | Concentration of FADH2 (reduced) | 29 | |
| [FAD] | 0.01 | mM | Concentration of FAD (oxidized) | 29 | |
| pa | 1.656 10−5 | s−1 | Sum of products of rate constants | 31,32 | g,h |
| pb | 3.373 10−7 | s−1 | Sum of products of rate constants | 32 | g,h |
| pc1 | 9.651 10−14 | s−1 | Sum of products of rate constants | 31 | g,h |
| pc2 | 4.585 10−14 | s−1 | Sum of products of rate constants | 31 | g,h |
| p1 | 1.346 10−8 | Sum of products of rate constants | 31,32 | g,h | |
| p2 | 7.739 10−7 | Sum of products of rate constants | 31,32 | g,h | |
| p3 | 6.65 10−15 | Sum of products of rate constants | 31,32 | g,h | |
| ρF1 | 0.06–1.8 | mM | Concentration of F1F0-ATPase | 31,32 | g,h |
| KF1 | 1.71 106 | Equilibrium constant of ATP hydrolysis | 33 | g,h | |
| R | 8.315 | V C mol−1 °K−1 | Gas constant | ||
| T | 310.16 | °K | Mammalian body temperature | ||
| F | 96480 | C mol−1 | Faraday constant | ||
| Pi | 20.0 | mM | Inorganic phosphate concentration | 33 | i |
| Cm | 15.0 | mM | Total sum of mito adenine nucleotides | 34 | d |
| VmaxANT | 0.05–24.0 | mM s−1 | Maximal rate of the ANT | 35 | g,h |
| h | 0.5 | Fraction of ΔΨm | 35 | g,h | |
| [ADP]i | 0.05–0.2 | mM | Cytoplasmic ADPi concentration | 35 | |
| [ATP]i | 6.5 | mM | Cytoplasmic ATPi concentration | 35 | d |
| gH | 0.01 | mM s−1 V−1 | Ionic conductance of the inner membrane | 36 | g,h |
| ΔpH | −0.6 | pH units | pH gradient across the inner membrane | 37 | k |
| CPN | 10.0 | mM | Total sum of mito pyridine nucleotides | 30 | b |
| Cmito | 1.812 | mM V−1 | Inner membrane capacitance | 2 | l,m |
| Model parameters: calcium dynamics | |||||
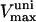 |
0.625–1.25 | μM s−1 | Vmax uniport Ca2+ transport | 38 | g,h |
| [Ca2+]i | 2.0 10−2–1.2 | μM | Cytosolic Ca2+ concentration | 38 | i |
| ΔΨ° | 0.091 | Volts | Offset membrane potential | 38 | g,h |
| Kact | 3.8 10−4 | mM | Activation constant | 38 | g,h |
| Ktrans | 0.019 | mM | Kd for translocated Ca2+ | 38 | g,h |
| L | 110.0 | Keq for conformational transitions in uniporter | 38 | g,h | |
| na | 2.8 | Uniporter activation cooperativity | 38 | g,h | |
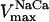 |
0.005–0.2 | mM s−1 | Vmax of Na+/Ca2+ antiporter | 39 | g,h |
| b | 0.5 | ΔΨm dependence of Na+/Ca2+ antiporter | 39 | g,h | |
| KNa | 9.4 | mM | Antiporter Na+ constant | 39 | g,h |
| [Na+]i | 10.0 | mM | Cytosolic Na+ concentration | 39 | g,h |
| KCa | 3.75 10−4 | mM | Antiporter Ca2+ constant | 39 | g,h |
| n | 3 | Na+/Ca2+ antiporter cooperativity | 39 | g,h | |
| f | 0.0003 | Fraction of free [Ca2+]m | 12 | g,h |
References: a, Dudycha et al., 2000; b, Albe et al., 1990; c, calculated from Jeffrey et al., 1999; d, Corkey et al., 1986; e, Singer, 1966; f, adjusted; g, Magnus and Keizer, 1997; h, Magnus, 1995; i, Crompton, 1999; j, calculated from data of Emyanitoff and Kelly, 1982; k, Jung et al., 1989; l, Gunter and Pfeiffer, 1990; m, Wojtczak et al., 1986.
