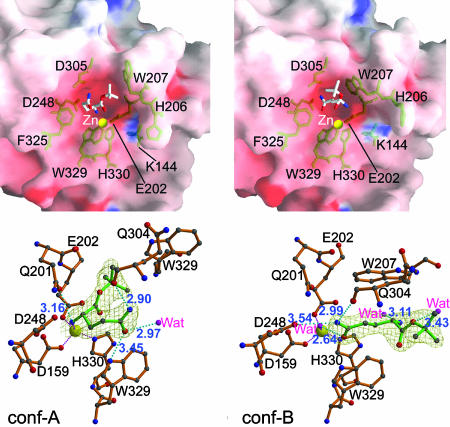
Fig. 3.
Comparison of conf-A (Left) and conf-B (Right) of the mutant E201Q bound to the substrate glutamine t-butyl ester. (Upper) Properties of the molecular surface. The charge potentials are calculated by using grasp (27) with a range of –20 to +20 kBT (where kB is the Boltzmann constant and T is temperature in kelvin), colored from red to blue. The stick models for substrate and some active-site residues are colored white and green, respectively. The zinc ions are shown as yellow balls. Note that the different orientations of His-206 and Trp-207 in conf-A and conf-B result in the different active-site conformations and different substrate-binding modes. (Lower) A close-up view of the binding of glutamine t-butyl ester to the active site. The substrate and human QC residues are represented with a ball-and-stick model colored green and orange, respectively. The 2Fo – Fc electron density maps (contoured at 1.0σ) for substrate are shown. Dotted lines in cyan and magenta depict the hydrogen bonds (distance is in Å) and coordination bonds, respectively.