Abstract
Peripheral tolerance can be achieved in many but not all murine allograft models. The requirements for controlling more aggressive immune responsiveness and generating peripheral tolerance in stringent allograft models are unknown. Understanding these requirements will provide insight toward ultimately achieving tolerance in humans, which are also resistant. We now demonstrate that the combination of donor-specific transfusion, anti-CD45RB, and anti-CD154 uniformly achieves >90-d survival of BALB/c skin allografts on C57BL/6 recipients. Recipients exhibit marked hyporesponsiveness to alloantigen in vitro. In distinct contrast to less rigorous models, engraftment remains absolutely dependent on cytotoxic T lymphocyte antigen 4 signaling, even after grafts are healed, suggesting that prolonged engraftment cannot simply be attributed to more effective depletion of alloreactive T cells but is actively maintained by regulation. Concordantly, we show that both CD4 and CD8 regulatory cells are required and can transfer donor-specific tolerance to naïve recipients. Nonetheless, most recipients ultimately develop gradual graft loss (median survival time = 140 d), suggesting that alloreactive cells emerging from the thymus eventually overwhelm regulatory capacity. In agreement, adding thymectomy to the regimen results in permanent engraftment (>250 d) and donor-specific tolerance not observed previously in this model. These results highlight the potency of both CD4 and CD8 regulatory cells but also suggest that in stringent settings, regulatory T cell longevity and capacity for infectious tolerance compete with prolonged graft immunogenicity and thymic output. These results provide insight into the mechanisms of tolerance in stringent models and provide a rational basis for innovative tolerogenic strategies in humans.
Keywords: transplantation, rodent, CD4 cell, CD8 cell, regulatory T cell
Immunological tolerance remains an unfulfilled goal in clinical transplantation. Although peripheral tolerance can be achieved in many rodent transplant models, others remain resistant. The degree of resistance to tolerance depends on the type of allograft, the extent of allogeneic disparity, and poorly defined genetic factors encoded outside of the MHC (1–4). Together, these factors determine allograft immunogenicity, responding clone size, and type and aggressiveness of the immune response.
Because the immunological response differs, the requirements for achieving tolerance vary with stringency of the model (1–4). Whereas T helper 2 deviation alone may interrupt autoimmunity or prevent allograft rejection across minor mismatches, prolonged survival of MHC disparate allografts requires T cell deletion to trim the relatively large responding clone size (1, 5–7). In fully allogeneic skin transplantation, alloaggressive CD8 cells play an important role in rejection, particularly in genetic high-responder strains like C57BL/6 (3, 4, 8, 9). Such CD8 cells are generally resistant to costimulatory blockade, and prolongation of graft survival requires therapeutic agents that directly target CD8 cells [such as anti-CD8 mAbs or donor-specific transfusion (DST)], in addition to agents that alter CD4 responses (3, 4, 9, 10). Yet, these approaches remain insufficient, and thus the requirements for achieving tolerance in stringent skin graft models remain unclear.
CD4+ regulatory T cells (Tregs) are generated in many transplant models and may contribute to maintaining tolerance (11–13). However, their role in stringent allograft models is not well established. For example, CD4 CD25+ Tregs from tolerant, fully allogeneic skin graft recipients could adoptively transfer tolerance to immunodeficient recipients; however, tolerance was neither donor-specific nor cytotoxic T lymphocyte antigen 4 (CTLA-4)-dependent (10). Moreover, similar numbers of CD25+ Tregs with the same activity were also found in naïve mice, nontolerant allograft recipients, and mice with established mixed chimerism. Thus, these cells were not specifically induced, and their role in maintaining primary skin graft survival is unclear. In a more stringent strain combination where peripheral tolerance had not been achieved, CD4 cells were required to maintain skin graft survival (14). However, these cells did not exhibit regulatory activity, and subsequent studies revealed that the essential role for CD4 cells in this model relates to an important role in CD8 cell depletion (14, 15).
To study the requirements of peripheral tolerance in stringent transplant models, we used a synergistic combination of anti-CD45RB, anti-CD154, and DST, which allowed markedly prolonged engraftment in all mice using highly resistant C57BL/6 recipients of BALB/c skin grafts. Unlike the case in less stringent models, blocking anti-CTLA-4 mAbs could precipitate late rejection of skin grafts, indicating that alloreactive cells were being held in check by Tregs. Adoptive transfer studies revealed that both CD4 cells and CD8 cells exhibited Treg activity. In agreement, graft survival was shortened in both CD4- and CD8-deficient recipients. Ultimately, most mice treated with this regimen developed rejection, suggesting that the balance between Tregs and alloreactive T cells emerging from the thymus was eventually tipped. Concordantly, addition of thymectomy to this regimen allowed permanent engraftment and long-term donor-specific tolerance not previously achieved in this stringent allograft model.
Methods
Mice. C57BL/6 (H-2b), C57BL/6-CD4tmlMak (CD4 KO; H-2b) (in which KO indicates knockout), C57BL/6-CD8αtmlMak (CD8 KO; H-2b), and recombination activating gene (Rag) 1-deficient (Rag KO; C57BL/6) recipients and C3H (H-2k) donors (6–8 weeks old) were from The Jackson Laboratory. BALB/c (H-2d) donors were from Taconic Farms.
Antibodies. Anti-CD45RB (MB23G2, American Type Culture Collection), anti-CD154 (MR1, a kind gift from R. Noelle, Dartmouth Medical Center, Lebanon, NH), and anti-CTLA-4 (4F10, a kind gift from J. Bluestone, University of California, San Francisco) were produced commercially (Bioexpress, West Lebanon, NH). Anti-CD8 (2.43, American Type Culture Collection) was previously grown as ascites.
Treatment Protocols. As indicated, recipients received anti-CD45RB (100 μg i.v. on d -1, 0, 1, 2, 5, and 8), anti-CD154 mAb (250 μg i.p. on d 0, 2, 4, 6, and 8), or anti-CTLA-4 mAb (500 μg i.p. starting on d 0 or d 30, followed by 250 μg i.p. every other day for five doses). Where indicated, CD8 cells were depleted (>95%) with anti-CD8 ascites (0.1 ml i.p.; ≈100 μg of purified mAb) 6, 3, and 1 d before transplantation as described in ref. 16 and weekly thereafter. DST consisted of 5 × 106 BALB/c splenocytes i.v. on the day of skin transplantation.
Surgical Procedures. Full-thickness BALB/c skin was grafted to the dorsum of C57BL/6 recipients as described in ref. 17. Rejection was defined as complete loss of viable skin on visual inspection. As indicated, C57BL/6 mice were thymectomized by suction that was applied through a sternotomy.
Cell Purification and Adoptive Transfer. C57BL/6 recipients of BALB/c skin were treated with DST plus anti-CD45RB and anti-CD154. After 40 d, CD4 or CD8 cells from lymph nodes and spleen were purified by positive selection by using MACS isolation kits (Miltenyi Biotec, Auburn, CA). Cell purity was >98% by flow cytometry. Isolated cells were adoptively transferred (i.v.) with or without splenocytes from naïve C57BL/6 mice into C57BL/6 Rag KO mice that had received a heterotopic BALB/c cardiac allograft 1 d earlier, as described in ref. 16. Rejection of cardiac allografts was defined as cessation of a palpable beat and was confirmed by direct visualization after laparotomy (16).
Enzyme-Linked Immunospot (ELISPOT). ELISPOT was performed as described in ref. 16. Briefly, immunospot plates (Cellular Technology, Cleveland) were coated with anti-mouse IFN-γ (4 μg/ml). Recipient splenocytes plus irradiated (3,000 rad) syngeneic or allogeneic splenocytes (106 each) were cultured for 24 h, incubated with biotinylated anti-mouse IFN-γ (2 μg/ml) and streptavidin horseradish peroxidase (DAKO), and developed with 3-amino-9-ethylcarbazole (Sigma-Aldrich). The resulting spots were counted on a computer-assisted immunospot image analyzer (T Spot Image Analyzer, Cellular Technology). mAbs were from Pharmingen.
Serum Alloantibody. Serial dilutions of sera from C57BL/6 recipients of BALB/c skin grafts were added to naïve BALB/c splenocytes, followed by anti-mouse Ig secondary Ab. Test samples were compared with isotype-matched controls, and the relative median fluorescence was determined.
Results
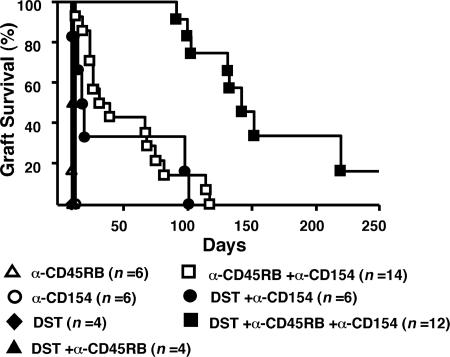
DST Dramatically Enhances Skin Graft Survival Induced by Anti-CD45RB Plus Anti-CD40L in a Stringent Allograft Model. We previously demonstrated synergy between anti-CD45RB and anti-CD154 in skin transplantation (17). Whereas individually these agents do not prolong engraftment, combined therapy allows 40% of allografts to survive >65 d and 15% of allografts to survive >100 d (Fig. 1). Previous reports indicated that anti-CD154 plus DST could also significantly prolong skin graft survival in this strain combination (14). Here, the addition of DST to anti-CD40L increased MST from 14 to 20 d; however, two of six recipients exhibited graft survival for almost 100 d (Fig. 1). Based on this synergy, we added DST to anti-CD45RB plus anti-CD154. This combination dramatically enhanced skin engraftment, with all recipients surviving >90 d (MST = 140 d; Fig. 1). Although this regimen induces long-term survival, most allografts were subject to late indolent rejection, and only 20% of the allografts remained healthy at 250 d (Fig. 1). Nonetheless, this approach greatly prolonged graft survival and provided an opportunity to study the mechanisms involved in anticipation of understanding the requirements for achieving peripheral tolerance.
Fig. 1.
DST enhances graft survival mediated by anti-CD154 and anti-CD45RB. Recipient mice were treated as indicated (see Methods for details). DST plus anti-CD45RB did not enhance graft survival over anti-CD45RB alone. In contrast, DST plus anti-CD40L significantly prolonged graft survival over anti-CD154 alone (P = 0.025). DST was most effective in combination with both anti-CD45RB mAb and anti-CD154 mAb (MST = 140 d; P < 0.0001, with vs. without DST).
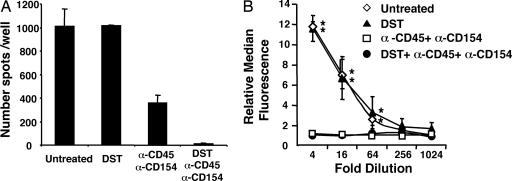
Significant Loss of Alloreactivity Induced by DST Plus Anti-CD45RB mAb and Anti-CD154 Therapy. To determine the effects of combination therapy on in vivo T cell priming, we examined the frequency of IFN-γ-producing donor-specific T cells in skin allograft recipients by enzyme-linked immunospot on d 10 (16). The frequency of alloreactive T cells after DST/anti-CD45RB/anti-CD154 treatment was extremely low and significantly lower than after DST alone or anti-CD45RB plus anti-CD154 (Fig. 2A). This decrease is likely to be important in promoting long-term engraftment in this setting, because rejection of skin grafts correlates with increased IFN-γ production by T cells from stringent recipients like C57BL/6 (4). Thus, anti-CD45RB plus anti-CD154 significantly inhibit alloresponsive T cells, and inhibition is markedly enhanced by addition of DST.
Fig. 2.
Effect of DST, anti-CD45RB, and anti-CD154 treatment on alloreactive T and B cells. (A) Frequency of IFN-γ-producing donor-specific T cells in C57BL/6 recipients 10 d after transplantation with BALB/c skin. Recipients were treated as indicated. Recipient splenocytes (106) were incubated with irradiated donor splenocytes, and frequency of IFN-γ-producing cells was determined by enzyme-linked immunospot. Data are expressed as mean ± SEM of triplicate wells and are representative of four independent experiments. The frequency after DST plus anti-CD45RB plus anti-CD154 treatment was consistently lower than other groups (P < 0.0001). (B) Circulating alloantibody in C57BL/6 recipients after BALB/c skin transplantation. Naïve BALB/c splenocytes were incubated with 50 μl of serially diluted sera from recipient mice on d 10. Cells were then incubated with anti-mouse IgG1 fluorochrome conjugate vs. an isotype-matched control antibody. The median fluorescence of each sample was compared by flow cytometry. Data are means ± SEM from three mice. *, P < 0.05 vs. anti-CD45RB plus anti-CD154 with or without DST.
To assess B cell-mediated humoral alloimmune responses, we measured alloantibody production by flow cytometry (18, 19). Whereas untreated recipients or recipients treated with DST alone mounted a significant alloantibody response, anti-CD45RB plus anti-CD154 treatment (with or without DST) significantly inhibited alloantibody production (Fig. 2B). These studies demonstrate that this combination regimen prevents significant T and B cell priming after skin transplantation.
Role of CTLA-4 in Long-Term Allograft Acceptance. The marked decrease in T cell priming after combination therapy could be due to depletion of alloreactive clones. Both DST an anti-CD40L have both been shown to act at least in part through depletion of CD8 and CD4 cells (9, 20, 21). To determine whether complete deletion explains the decreased alloreactivity and improved graft survival observed after combination therapy, we assessed the effect of CTLA-4 blockade. CTLA-4-mediated negative signals appear to be essential for tolerance induction by various peripheral strategies (14, 16, 22–25). Indeed, if alloreactive T cells have been deleted, anti-CTLA-4 should not precipitate rejection by augmenting the T cell response. Treatment with anti-CTLA-4 in the peritransplant period resulted in acute rejection in five of six recipients (Fig. 3; MST = 22.5 d).
Fig. 3.
CTLA-4 signaling plays a key role in induction and maintenance of long-term allograft acceptance by DST, anti-CD45RB, and anti-CD154. Recipient mice were treated with anti-CTLA-4 mAb initiated on d 0 or on d 30 after transplantation, as detailed in Methods. Both early and late CTLA-4 blockade triggered allograft rejection (MST = 23 d and 75 d, respectively). * and **, P < 0.0001 vs. DST plus anti-CD45RB plus anti-CD154.
Although CTLA-4 signaling has been shown to be critical for initial engraftment in various transplant models, CTLA-4 blockade does not precipitate rejection during the maintenance phase of transplant tolerance to vascularized cardiac allografts (16, 23, 24). In contrast, administration of anti-CTLA-4 to recipients of well healed skin allografts (starting on d 30) results in rejection in all recipients (MST = 75 d; Fig. 3). Thus, in the setting of a more stringent allograft, CTLA-4 signaling is critical not only for induction but also for maintenance of long-term allograft survival. Although deletion may contribute to the hyporesponsiveness and prolonged allograft survival that is observed, alloreactive T cells are still present but are apparently kept in check by active regulatory mechanisms. Importantly, anti-CTLA-4 interferes with Treg function (26–28). Taken together, these results suggest that potentially alloreactive T cells are still present and respond once released from active control by Tregs.
Generation of CD4+ and CD8+ Tregs by DST/Anti-CD45RB/Anti-CD154 Treatment. To directly address whether Tregs were generated by treatment with DST/anti-CD45RB/anti-CD154, we used an adoptive transfer model (11–13). C57BL/6 recipients of BALB/c skin grafts received combination therapy. Forty days later, 5 × 106 splenic mononuclear cells from these mice were adoptively transferred into C57BL/6 Rag KO recipients that had received a BALB/c heart allograft 1 d earlier. As shown in Table 1, adoptive transfer of 5 × 106 naïve C57BL/6 splenocytes into Rag KO mice resulted in prompt rejection (MST = 9 d). In contrast, the same number of C57BL/6 splenocytes transferred from treated skin graft recipients did not precipitate rejection in any recipients (MST > 100 d). Importantly, when Rag KO recipients were reconstituted with equal numbers of C57BL/6 splenocytes from both naïve mice and treated skin graft recipients, allograft rejection did not occur. Furthermore, adoptively transferred splenocytes from treated mice rapidly rejected third-party (C3H) allografts (MST = 9 d). These data indicate that T cells from treated mice are immunocompetent and that rejection is being prevented by donor-specific Tregs. Importantly, upon fractionation, we found that both CD4 and CD8 cells from transplanted mice treated with DST/anti-CD45/anti-CD154 demonstrate regulatory activity (Table 1). In contrast to CD4+ Tregs, the in vivo role of regulatory CD8 cells in allograft models is not well understood.
Table 1. CD4 and CD8 Treg generation after DST, α-CD45RB, and α-CD154 treatment.
| Donor | Adoptive transfer* | n | MST, d |
|---|---|---|---|
| BALB/c | Naïve | 5 | 9 |
| BALB/c | Treated | 4 | >100 |
| C3H | Treated | 4 | 10 |
| BALB/c | Naïve plus treated | 4 | >100 |
| BALB/c | Naïve plus treated CD4+ | 5 | >100 |
| BALB/c | Naïve plus treated CD8+ | 4 | >100 |
A total of 5 × 106 cells from naïve C57BL/6 mice and/or from treated C57BL/6 skin graft recipients
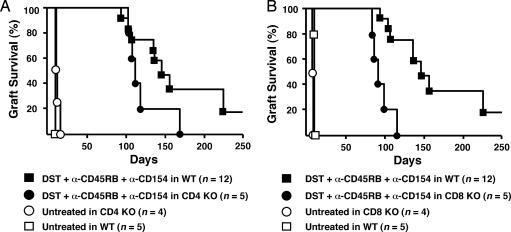
Role of DST/Anti-CD45RB/Anti-CD154 in CD4- and CD8-Deficient Mice. To more directly address the role of CD4 and CD8 cells in prolonged allograft survival, we used C57BL/6 CD4 KO or CD8 KO mice as skin allograft graft recipients. Similar to WT mice, untreated CD4 and CD8 deficient recipients promptly reject BALB/c skin allografts (MST 13d and 9d, respectively). As shown (Fig. 4), combination therapy did significantly prolong skin graft survival in both CD4 and CD8 KO mice. However, long-term skin allograft survival in CD4 KO recipients was significantly worse than in WT recipients treated with the same regimen (MST = 108 vs. 140 d, respectively; Fig. 4A). Moreover, treated CD8 KO recipients also exhibited a significant decrease in long-term engraftment compared with WT mice (MST = 92 d vs. 140 d, respectively; Fig. 4B). These data support the notion that both CD8 and CD4 cells with regulatory activity play an important role in maintaining long-term allograft survival in this model.
Fig. 4.
Allograft survival in CD4- and CD8-deficient mice treated with combination therapy. C57BL/6 CD4 (A) and CD8 (B) KO recipients were treated with DST/anti-CD45/anti-CD154. (A) Untreated graft survival in CD4 KO recipients was nominally prolonged compared with WT recipients (MST = 12.5 vs. 11 d; P = 0.007). However, WT recipients exhibit significantly better long-term graft survival (>120 d) than CD4 KO recipients after combination therapy (P = 0.035). (B) Treated CD8 KO mice exhibited prolonged engraftment (MST = 92 vs. 9 d for untreated CD8 KO controls; P < 0.005). However, WT recipients exhibit significantly better graft survival than CD8 KO recipients after combination therapy (P = 0.0003).
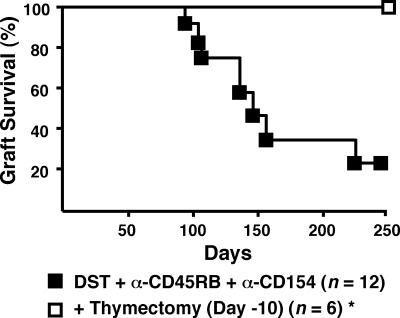
Addition of Thymectomy Leads to Donor-Specific Peripheral Tolerance. DST/anti-CD45RB/anti-CD154 treatment has a remarkable affect on allograft survival in this stringent model. None of the grafts were lost for >90 d, and, moreover, when rejection occurred, graft loss in individual mice was slowly progressive. Thus, in terms of timing and tempo, skin graft rejection was delayed and chronic. Taken together, the data suggest that regulatory activity either fades over time and/or that new alloreactive T cells emerging from the thymus gradually overwhelm the capacity of regulatory cells to prevent rejection. To test the latter possibility, we performed thymectomy on mice before skin transplantation and treatment with DST/anti-CD45RB/anti-CD154. As shown in Fig. 5, all such recipients accepted grafts indefinitely (MST > 250 d). Remarkably, all of these mice also accepted donor strain second skin allografts (MST > 100 d) but acutely rejected third-party skin allografts (MST = 12 d; Table 2). In both settings, the initial skin graft was preserved.
Fig. 5.
Addition of thymectomy to DST, anti-CD45RB, and anti-CD40L leads to permanent engraftment in all recipients. Recipients were thymectomized 10 d before transplantation as described in Methods. *, P = 0.018, graft survival of thymectomized vs. euthymic mice.
Table 2. Donor-specific tolerance to second skin grafts > 100 d after placement of first skin graft in thymectomized recipients initially treated with DST, α-CD45RB, and α-CD154.
| First graft | Recipient | Second graft | n | MST, d |
|---|---|---|---|---|
| BALB/c | C57BL/6 | BALB/c | 5 | >100 |
| BALB/c | C57BL/6 | C3H | 5 | 12 |
Discussion
Stringent skin allografts are highly resistant to the induction of peripheral tolerance, and thus the requirements for tolerance remain unclear. Indeed, it has been suggested that peripheral tolerance mechanisms may not be sufficiently robust and that only central deletion (i.e., after bone marrow replacement or mixed chimerism) can ensure robust tolerance in this setting (29). We now show that a peripheral strategy employing DST, anti-CD45RB, and anti-CD154 markedly prolongs allograft survival in C57BL/6 recipients of BALB/c skin grafts, with some allografts surviving >250 d. Long-term engraftment was associated with marked hyporesponsiveness to donor alloantigen and requires both CD4 and CD8 Tregs. Addition of thymectomy converted prolonged engraftment into robust donor-specific peripheral tolerance, not previously reported in this stringent model. These data are consistent with the notion that alloreactive cells emerging from the thymus accumulate and eventually overwhelm regulatory capacity.
Both CD25+ and CD25- CD4 Tregs have been shown to play a role in allograft tolerance (11–13). One difficulty with the use of CD25 as a marker for Tregs is lack of specificity. Indeed, up to 40–50% of natural (Foxp3+) Tregs in spleen and tissues are CD25- (30). Tregs can prevent adoptively transferred naïve cells from rejecting allografts, prevent rejection of second allografts sharing only one haplotype with the original allograft (linked suppression), adoptively transfer tolerance to naïve recipients, and induce naïve cells to gain regulatory activity (infectious tolerance). However, the actual requirement for Tregs in maintaining primary allograft survival is not well defined. The role of CD4 Tregs in stringent skin graft models is considerably less clear. As noted previously, equivalent CD4+ CD25+“natural” Tregs that are capable of adoptively transferring tolerance to new hosts could be obtained from naïve mice and from both tolerant and nontolerant recipients of fully allogeneic skin transplants (10). In the same stringent strain combination used here, prolonged skin graft survival in the absence of tolerance was not associated with regulatory CD4 cells (14, 15).
The current study indicates that CD4+ Tregs do play an important role in maintaining graft survival in a stringent setting where tolerance is achieved. The origin of these Tregs (induced or natural) and phenotype (e.g., Foxp3 and CD25 expression) will require further investigation. CTLA-4 is a potent inhibitor of T cell activation. Both induced and natural CD4+ Tregs express CTLA-4, which is also induced by anti-CD45RB treatment (22, 31–33). In contrast to effector T cells, CTLA-4 blockade appears to directly inhibit Tregs, which augments autoimmunity in several models (26–28). Another interpretation of these data is that under the influence of CTLA-4 blockade, effector cells are less subject to regulation by CD4 Tregs. Either way, our data indicate that Tregs are required to keep effector cells in check even after stable engraftment. This finding is distinctly different from the case in less stringent allograft models, where anti-CTLA-4 does not precipitate rejection unless given in the peritransplant period, even when the same therapeutic agents are involved (16, 23, 24). The ability of CD4 Tregs to maintain engraftment in such stringent models demonstrates their potency.
Although suppressor T cells were initially described as being CD8+ (34), much of the renewed interest in regulation has focused on CD4 cells. However, several recent studies provide insight into CD8+ Tregs and are relevant to alloimmunity. Human CD28- CD8 cells isolated after multiple rounds of in vitro allogeneic stimulation suppress CD4 proliferation in vitro by inhibiting antigen-presenting cell maturation (35). In addition, human CD8+ Tregs can be generated by culturing with allogeneic plasmacytoid dendritic cells (36). These cells inhibit allospecific proliferation of naïve CD8 cells through IL-10 secretion. Nevertheless, the in vivo role of CD8+ Tregs in allograft tolerance is not well defined. There exists a single report that rats made tolerant by administration of UV-irradiated donor splenocytes starting 3 weeks before cardiac transplantation harbor CD8 cells capable of adoptively transferring tolerance in 60% of irradiated naïve cardiac allograft recipients (37). However, the role of CD8 Tregs in stringent allograft models using a clinically applicable therapeutic regimen has not been previously described. The importance of CD8 Tregs in maintaining graft survival in the current model is suggested by their ability to transfer tolerance to immunodeficient mice and the decreased graft survival noted in CD8 KO mice. This involvement of CD8 Tregs is important because CD8 effectors have been shown to play a key role in the rejection of stringent skin grafts. This dual role may explain why attempts to induce tolerance to stringent skin grafts by CD8 cell depletion have been unsuccessful (3, 4). Further characterization of the phenotype of CD8 Tregs involved in skin graft tolerance (e.g., CD28 and Foxp3 expression) will be required to determine whether these cells can be separated from CD8 effector cells, which may have therapeutic implications, as noted above.
The pattern of rejection after combination therapy suggests additional differences in the immune response to stringent allografts. The rejection of skin grafts after such a marked delay and its prevention by thymectomy distinguish this model from other rodent allograft models where graft survival of 100–120 d signifies permanent engraftment in euthymic recipients. It is known that “danger” signals after skin transplantation persist long term (38). In the current setting, addition of thymectomy (which prevents emergence of new naïve effector cells after therapeutic agents have disappeared) results in long-term donor-specific tolerance. Taken together, these data suggest that nascent alloreactive cells from the thymus accumulate until they ultimately overwhelm the capacity of Tregs to maintain tolerance or induce new regulatory cells. Even if Treg activity does diminish over time, tolerance is only broken when new effector cells can arise (through an intact thymus). Thus, we conclude that accumulation of new effector cells is the predominant mechanism by which late rejection occurs. Moreover, these data suggest that Tregs and infectious tolerance have their limits in the face of inherently aggressive effector cells in genetic high-responder strains. That late rejection does not occur in less stringent allograft models suggests that danger signals do not persist for as long and/or that less aggressive effector cells are insufficient to overwhelm regulatory capacity. On a practical level, late rejection indicates that reports of long-term skin graft survival based on observation of <150–200 d must be viewed with caution.
Finally, the requirements for tolerance in this model may provide insight into tolerance induction in primates, which are also highly resistant. On the one hand, the thymus is much less active in adult humans than in young mice, possibly obviating the need for thymectomy. On the other hand, the time scale required for maintaining “tolerance” is much longer. Thus, robust tolerance in mice might translate into shorter-term “operational tolerance” in humans that may require periodic retreatment to reestablish the balance between regulatory and effector cells.
In summary, these findings further our understanding of both the alloimmune response and requirements for tolerance to stringent allografts. Both CD4 and CD8 Tregs are required to maintain tolerance in this setting, demonstrating the extent of their potency. In addition to establishing the role of CD4 Tregs in stringent models, we demonstrate that ongoing CTLA-4 signaling is required to maintain “allograft homeostasis.” An aggressive CD8 response has been viewed as a major hurdle to skin transplantation. Although relatively little is known about alloreactive CD8+ Tregs in vivo, CD8 cells with in vitro regulatory activity have been detected in the peripheral blood of stable cardiac allograft recipients (39). Our data provide additional impetus to understand how these cells are induced and to rationally design therapeutic strategies that will limit alloaggressive CD8 cells but spare the regulatory subset. Despite the presence of potent Tregs in stringent allograft models, infectious tolerance and/or Treg durability appear to have their limitations. We speculate that in adult primates, maintaining robust and long-lived peripheral tolerance will depend on a balance between regulatory cells, thymic activity, and persistence of danger signals and, as in murine models, will depend on recipient age, genetic makeup, allogeneic disparity, and type of allograft. Indeed, tolerogenic regimens may require individualization. Moreover, thymectomy or periodic retreatment to reestablish the balance between regulatory and effector cells may be required.
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01 AI045485 (to D.M.R.), P01 AI056299, P01 AI41521, and R01 AI 51559 (to M.H.S.) and the Juvenile Diabetes Research Foundation (X.X.Z.).
Author contributions: T.B.S., G.P.B., M.H.S., and D.M.R. designed research; M.S., K.K., H.H., M.L., A.S.-F., A.Y., and X.X.Z. performed research; M.S., T.B.S., G.P.B., M.H.S., and D.M.R. analyzed data; and M.H.S. and D.M.R. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MST, median survival time; DST, donor-specific transfusion; Treg, regulatory T cell; KO, knockout; GS, graft survival; CTLA-4, cytotoxic T lymphocyte antigen-4.
References
- 1.Kishimoto, K., Sandner, S., Imitola, J., Sho, M., Li, Y., Langmuir, P. B., Rothstein, D. M., Strom, T. B., Turka, L. A. & Sayegh, M. H. (2002) J. Clin. Invest. 109, 1471-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips, N. E., Markees, T. G., Mordes, J. P., Greiner, D. L. & Rossini, A. A. (2003) J. Immunol. 170, 3015-3023. [DOI] [PubMed] [Google Scholar]
- 3.Trambley, J., Bingaman, A. W., Lin, A., Elwood, E. T., Waitze, S. Y., Ha, J., Durham, M. M., Corbascio, M., Cowan, S. R., Pearson, T. C. & Larsen, C. P. (1999) J. Clin. Invest. 104, 1715-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams, M. A., Trambley, J., Ha, J., Adams, A. B., Durham, M. M., Rees, P., Cowan, S. R., Pearson, T. C. & Larsen, C. P. (2000) J. Immunol. 165, 6849-6857. [DOI] [PubMed] [Google Scholar]
- 5.Li, Y., Li, X. C., Zheng, X. X., Wells, A. D., Turka, L. A. & Strom, T. B. (1999) Nat. Med. 5, 1298-1302. [DOI] [PubMed] [Google Scholar]
- 6.Wells, A. D., Li, X. C., Li, Y., Walsh, M. C., Zheng, X. X., Wu, Z., Nunez, G., Tang, A., Sayegh, M., Hancock, W. W., Strom, T. B. & Turka, L. A. (1999) Nat. Med. 5, 1303-1307. [DOI] [PubMed] [Google Scholar]
- 7.Zheng, X. X., Sanchez-Fueyo, A., Sho, M., Domenig, C., Sayegh, M. H. & Strom, T. B. (2003) Immunity 19, 503-514. [DOI] [PubMed] [Google Scholar]
- 8.Iwakoshi, N. N., Mordes, J. P., Markees, T. G., Phillips, N. E., Rossini, A. A. & Greiner, D. L. (2000) J. Immunol. 164, 512-521. [DOI] [PubMed] [Google Scholar]
- 9.Iwakoshi, N. N., Markees, T. G., Turgeon, N., Thornley, T., Cuthbert, A., Leif, J., Phillips, N. E., Mordes, J. P., Greiner, D. L. & Rossini, A. A. (2001) J. Immunol. 167, 6623-6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graca, L., Le Moine, A., Lin, C. Y., Fairchild, P. J., Cobbold, S. P. & Waldmann, H. (2004) Proc. Natl. Acad. Sci. USA 101, 10122-10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood, K. J. & Sakaguchi, S. (2003) Nat. Rev. Immunol. 3, 199-210. [DOI] [PubMed] [Google Scholar]
- 12.Waldmann, H. & Cobbold, S. (2001) Immunity 14, 399-406. [DOI] [PubMed] [Google Scholar]
- 13.Graca, L., Thompson, S., Lin, C. Y., Adams, E., Cobbold, S. P. & Waldmann, H. (2002) J. Immunol. 168, 5558-5565. [DOI] [PubMed] [Google Scholar]
- 14.Markees, T. G., Phillips, N. E., Gordon, E. J., Noelle, R. J., Shultz, L. D., Mordes, J. P., Greiner, D. L. & Rossini, A. A. (1998) J. Clin. Invest. 101, 2446-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banuelos, S. J., Markees, T. G., Phillips, N. E., Appel, M. C., Cuthbert, A., Leif, J., Mordes, J. P., Shultz, L. D., Rossini, A. A. & Greiner, D. L. (2004) Transplantation 78, 660-667. [DOI] [PubMed] [Google Scholar]
- 16.Sho, M., Yamada, A., Najafian, N., Salama, A. D., Harada, H., Sandner, S. E., Sanchez-Fueyo, A., Zheng, X. X., Strom, T. B. & Sayegh, M. H. (2002) J. Immunol. 169, 3744-3751. [DOI] [PubMed] [Google Scholar]
- 17.Rothstein, D. M., Livak, M. F., Kishimoto, K., Ariyan, C., Qian, H. Y., Fecteau, S., Sho, M., Deng, S., Zheng, X. X., Sayegh, M. H. & Basadonna, G. P. (2001) J. Immunol. 166, 322-329. [DOI] [PubMed] [Google Scholar]
- 18.Lin, H., Rathmell, J. C., Gray, G. S., Thompson, C. B., Leiden, J. M. & Alegre, M. L. (1998) J. Exp. Med. 188, 199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada, A., Kishimoto, K., Dong, V. M., Sho, M., Salama, A. D., Anosova, N. G., Benichou, G., Mandelbrot, D. A., Sharpe, A. H., Turka, L. A., et al. (2001) J. Immunol. 167, 140-146. [DOI] [PubMed] [Google Scholar]
- 20.Monk, N. J., Hargreaves, R. E., Marsh, J. E., Farrar, C. A., Sacks, S. H., Millrain, M., Simpson, E., Dyson, J. & Jurcevic, S. (2003) Nat. Med. 9, 1275-1280. [DOI] [PubMed] [Google Scholar]
- 21.van Maurik, A., de St. Groth, B. F., Wood, K. J. & Jones, N. D. (2004) J. Immunol. 172, 2163-2170. [DOI] [PubMed] [Google Scholar]
- 22.Fecteau, S., Basadonna, G. P., Freitas, A., Ariyan, C., Sayegh, M. H. & Rothstein, D. M. (2001) Nat. Immunol. 2, 58-63. [DOI] [PubMed] [Google Scholar]
- 23.Judge, T. A., Wu, Z., Zheng, X. G., Sharpe, A. H., Sayegh, M. H. & Turka, L. A. (1999) J. Immunol. 162, 1947-1951. [PubMed] [Google Scholar]
- 24.Sanchez-Fueyo, A., Weber, M., Domenig, C., Strom, T. B. & Zheng, X. X. (2002) J. Immunol. 168, 2274-2281. [DOI] [PubMed] [Google Scholar]
- 25.Zheng, X. X., Markees, T. G., Hancock, W. W., Li, Y., Greiner, D. L., Li, X. C., Mordes, J. P., Sayegh, M. H., Rossini, A. A. & Strom, T. B. (1999) J. Immunol. 162, 4983-4990. [PubMed] [Google Scholar]
- 26.Read, S., Malmstrom, V. & Powrie, F. (2000) J. Exp. Med. 192, 295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi, T., Tagami, T., Yamazaki, S., Uede, T., Shimizu, J., Sakaguchi, N., Mak, T. W. & Sakaguchi, S. (2000) J. Exp. Med. 192, 303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salomon, B., Lenschow, D. J., Rhee, L., Ashourian, N., Singh, B., Sharpe, A. & Bluestone, J. A. (2000) Immunity 12, 431-440. [DOI] [PubMed] [Google Scholar]
- 29.Wekerle, T., Sayegh, M. H., Hill, J., Zhao, Y., Chandraker, A., Swenson, K. G., Zhao, G. & Sykes, M. (1998) J. Exp. Med. 187, 2037-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontenot, J. D., Rasmussen, J. P., Williams, L. M., Dooley, J. L., Farr, A. G. & Rudensky, A. Y. (2005) Immunity 22, 329-341. [DOI] [PubMed] [Google Scholar]
- 31.Ariyan, C., Salvalaggio, P., Fecteau, S., Deng, S., Rogozinski, L., Mandelbrot, D., Sharpe, A., Sayegh, M. H., Basadonna, G. P. & Rothstein, D. M. (2003) J. Immunol. 171, 5673-5677. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi, S. (2004) Annu. Rev. Immunol. 22, 531-562. [DOI] [PubMed] [Google Scholar]
- 33.Wraith, D. C., Nicolson, K. S. & Whitley, N. T. (2004) Curr. Opin. Immunol. 16, 695-701. [DOI] [PubMed] [Google Scholar]
- 34.Green, D. R., Flood, P. M. & Gershon, R. K. (1983) Annu. Rev. Immunol. 1, 439-463. [DOI] [PubMed] [Google Scholar]
- 35.Liu, Z., Tugulea, S., Cortesini, R. & Suciu-Foca, N. (1998) Int. Immunol. 10, 775-783. [DOI] [PubMed] [Google Scholar]
- 36.Gilliet, M. & Liu, Y. J. (2002) J. Exp. Med. 195, 695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, J., Liu, Z., Witkowski, P., Vlad, G., Manavalan, J. S., Scotto, L., Kim-Schulze, S., Cortesini, R., Hardy, M. A. & Suciu-Foca, N. (2004) Transplant Immunol. 13, 239-247. [DOI] [PubMed] [Google Scholar]
- 38.Anderson, C. C., Carroll, J. M., Gallucci, S., Ridge, J. P., Cheever, A. W. & Matzinger, P. (2001) J. Immunol. 166, 3663-3671. [DOI] [PubMed] [Google Scholar]
- 39.Chang, C. C., Ciubotariu, R., Manavalan, J. S., Yuan, J., Colovai, A. I., Piazza, F., Lederman, S., Colonna, M., Cortesini, R., Dalla-Favera, R. & Suciu-Foca, N. (2002) Nat. Immunol. 3, 237-243. [DOI] [PubMed] [Google Scholar]