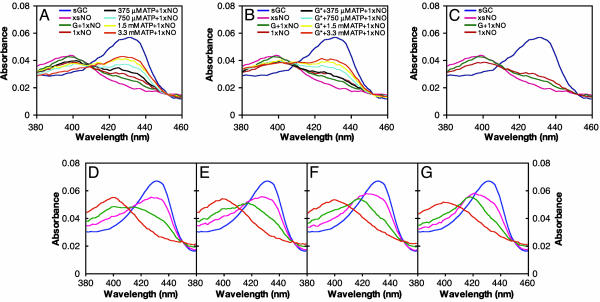
Fig. 4.
ATP inhibits the formation of the sGC heme-NO complex. sGC (400 nM) was premixed with no nucleotide, 30 μM GMPCPP, or 30 μM GMPCPP and 0 μM, 375 μM, 750 μM, 1.5 mM, or 3.3 mM ATP at 22°C. The same amount of PROLI/NO was added to all samples, which were then analyzed by absorption spectroscopy. Final spectra are shown. The spectra of ferrous sGC and sGC with excess NO (xsNO) are shown for comparison. (A) ATP prevents formation of ferrous-nitrosyl sGC, whereas GMPCPP (G*) increases formation. (B) The experiment shown in A was repeated with GMPCPP (G*) and ATP together. ATP prevents GMPCPP from increasing ferrous-nitrosyl formation. (C) GMPCPP (G*) accelerates formation of ferrous-nitrosyl sGC. (D) The effect of nucleotides on the binding of NO to the sGC heme at 4°C. sGC (400 nM) was premixed with 200 μM GTP, 200 μM GTP and 500 μM ATP (E), 200 μM GTP and 3 mM ATP (F), or 3 mM ATP (G). Excess PROLI/NO was added, and spectra were collected every 12 s to monitor NO-heme binding. The t = 0 spectrum is blue, followed by pink, green, and orange for t = 12, 24, and 36 s, respectively. Spectra acquired in the absence of nucleotide were the same as those acquired in the presence of 3 mM ATP (data not shown). In the presence of increasing amounts of ATP, the six-coordinate intermediate (λmax at 420 nm) is visible. Little intermediate is observed with GTP alone; ATP blocks GTP from accelerating the formation of the final five-coordinate NO-heme complex.