Abstract
Tebuconazole (TEB), a fungicide that inhibits 14α-demethylase (CYP51) and disrupts ergosterol synthesis, poses environmental and health risks due to its persistence and low biodegradability. This study examined TEB in vitro effects on rooster spermatozoa. In Experiment 1, semen from 10 Green-legged Partridge roosters was incubated with TEB (0, 0.1, 1, 10, 100 µM) at 36°C for 3 hours. Sperm motility was analyzed with Computer-Aided Sperm Analysis (CASA) system, while flow cytometry assessed membrane integrity, mitochondrial function, acrosome status, chromatin structure, intracellular calcium, apoptosis, caspase activity, and lipid peroxidation after 1 and 3 hours of exposure. Malondialdehyde (MDA) concentration and total antioxidant capacity (T-OAC) were measured by spectrophotometer. In Experiment 2, calcium channel blockers (SNX 325, MRS-1845, Nifedipine, HC-056456) were tested under the same conditions, focusing on motility, membrane integrity, calcium levels, apoptosis, caspase activity, and lipid peroxidation. Results in experiment 1 have shown that TEB (0.1, 1, 10 µM) reduced sperm velocity (VAP) after 3 hours (P < 0.01) without altering other motility parameters. Acrosome status, intracellular calcium level, and lipid peroxidation decreased significantly at all TEB concentrations (P < 0.01). Early apoptosis declined at 1 µM TEB (P < 0.01), while mitochondrial activity and membrane integrity remained stable. MDA levels were reduced (P < 0.01), with no effect on T-OAC. In Experiment 2, calcium channel blockers decreased motility parameters (VAP, VCL, VSL, MOT, PROG) and intracellular calcium levels (P < 0.01), but did not affect membrane integrity. Lipid peroxidation and caspase activity declined (P < 0.01), with no impact on early apoptosis. These findings underscore TEB's role in inhibiting calcium channels, reducing ion influx, blocking calcium-driven pore formation, thereby preserving membrane integrity. This mechanism mitigates early apoptosis and lipid peroxidation in chicken sperm, shedding light on TEB's impact on motility, calcium balance, and cell function.
Keywords: Tebuconazole, Chicken, Spermatozoa, Pesticide, Calcium homeostasis
Introduction
Tebuconazole (TEB) as a triazole fungicide, is widely used particularly in agriculture, for controlling fungal growth in crops. There are concerns that its persistence in the environment and low biodegradability may still pose a potential risk to human and animal health. Tebuconazole inhibits the enzyme 14α-demethylase (CYP51) (van den Bossche et al., 1978). CYP51 is a cytochrome P450 enzyme that catalyzes the C14 demethylation step in the sterol biosynthesis pathway. When this enzyme is inhibited by TEB or other azole fungicides, ergosterol cannot be synthesized properly, disrupting fungal cell membranes function (Chambers et al., 2013). As a result, fungi cannot maintain their cellular integrity, leading to cell damage and death (Kato et al., 1975).
Despite its effectiveness against fungal pathogens, several studies have reported endocrine-disrupting effects of TEB in humans and animals, leading to various reproductive and developmental toxicities. CYP51 is an evolutionarily conserved enzyme that is widely distributed across human tissues (EFSA, 2009), with notable expression in reproductive organs such as the testes and ovaries (Stromstedt et al., 1996). CYP51 plays a crucial role in the de novo synthesis of cholesterol by converting lanosterol and dihydrolanosterol into follicular fluid meiosis-activating sterol (FF-MAS), a key precursor of cholesterol (Keber et al., 2013). In the ovaries, CYP51 expression is stimulated by follicle-stimulating hormone (FSH) and helps protect granulosa cells during follicle maturation. Additionally, the deletion of the CYP51 gene in granulosa cells in mice results in the suppression of estradiol and progesterone secretion in response to FSH stimulation (Liu et al., 2017). All triazoles reduced the secretion of progesterone, estradiol, or both hormones at doses that, while not cytotoxic, exceed concentrations found in the environment. Importantly, these effects were also confirmed in studies on human cells (Serra et al. 2023a).
Research on TEB has demonstrated its ability to disrupt hormonal functions. In the study by Kjaerstad et al., 2010, it was reported that TEB exposure decreased estradiol and testosterone levels while increasing progesterone levels in vitro in the human adrenocortical carcinoma cell line. The toxicity of TEB also extends to developmental effects in offspring, with some studies indicating impacts on the development of reproductive organs, such as the testes and epididymis, as well as on sperm motility (Joshi et al., 2016). Additionally, TEB exposure has been associated with reductions in sperm count and quality, along with testicular dysfunction in male rats (Taxvig et al., 2007; Jacobsen et al., 2012). Furthermore, TEB has been shown to affect hormone production in both male and female fetuses, leading to long-term consequences for reproductive health (Taxvig et al., 2007). Collectively, these findings suggest that TEB may pose a significant risk to reproductive health, particularly through its hormonal and developmental effects.
The majority of triazole compounds disrupt steroidogenesis in chicken testes and reduce semen quality, partly by increasing oxidative stress (Serra et al., 2023b). However, it is unknown whether triazoles, including TEB, can affect acrosomal reaction and consequently fertility. The acrosome reaction in sperm plays a crucial role in oocyte fertilization (Baldi et al. 1996). During this process, the fusion of the sperm's plasma membrane with the outer acrosomal membrane occurs, resulting in the release of enzymes that enable sperm to penetrate the oocyte's protective layers. The essential element required for the occurrence of this reaction is calcium (Witte & Schäfer-Somi, 2007). Pesticides can have a significant negative impact on non-target organisms, depending on the specific substance and the level of exposure (Duzguner & Erdogan, 2010). Tebuconazole is also capable of contaminating the environment, as demonstrated in an experiment by Huang et al. (2022), where the aquatic environment contained TEB concentrations ranging from 0.001 ng/ml to 920 ng/ml. Our in vivo experimental results (not published yet) showed the presence of TEB in rooster blood at 29 ng/ml and in semen at 0.0888 ng/ml. These levels were observed in roosters fed with feed containing TEB at the maximum residual level (MRL), corresponding to 0.02256 mg/kg body weight per day, which is equivalent to 0.39% of the NOAEL. The doses used in the present experiment started at 0.1 µM, with 29 ng/ml corresponding to 0.0942 μM, and were subsequently increased tenfold to assess the potential toxic effects of TEB.
Therefore, we decided to investigate whether adding different doses of TEB (0 µM, 0.1 µM, 1 µM, 10 µM, 100 µM) in vitro to the rooster semen and incubating for 1 hour and 3 hours would result in impaired motility, acrosome status and increased oxidative stress. Following the example of other pesticides (Napierkowska et al., 2024), we also aimed to verify the effect of TEB on calcium signaling, which plays a key role in motility and the acrosome reaction.
Materials and methods
Chemicals
The chemicals used were purchased from Merck (St. Louis, MO, USA): SNX-325, catalog number: 508512; MRS 1845, catalog number: M1692; nifedipine, catalog number: 481981; Tebuconazole, catalog number: 32013; and PNA Alexa Fluor 488, lectin from Arachis hypogaea. The fluorescent dyes used included propidium iodide (PI), JC-1, Live/Dead Sperm Viability kit, Fluo 3 AM, C11-BODIPY581/591, AO (acridine orange), M540 (merocyanine), YO-PRO-1, CellEvent™ Caspase 3/7 Green Ready Flow™, Lipid Peroxidation (MDA) Assay Kit, Total Antioxidant Capacity (T-AOC) Colorimetric Assay Kit were obtained from Thermo Fisher Scientific (Waltham, MA, USA). CatSper Channel Blocker (HC-056456) was purchased from MCE (Monmouth Junction, NJ, USA).
Animals
The experiment involved Green-legged Partridge roosters, represented by 10 adult males housed individually in cages (70 cm × 95 cm × 85 cm) at a temperature of 18–20°C, under a 14-hour light and 10-hour dark photoperiod. The birds were fed a standard commercial feed for breeding flocks, without pesticide residues confirmed by Regional Experimental Station of the Institute of Plant Protection – NRI in Białystok. Water was available ad libitum. The study received an ethical opinion from the Local Animal Welfare Committee of the University of Life Sciences in Wroclaw (2K.2022).
Semen collection
Sperm was obtained twice a week using the dorso-abdominal massage technique described by Burrows and Quinn (1937). The procedure was consistently carried out by the same personnel and under identical conditions. All the obtained ejaculates were of high quality and suitable for research purposes. Within 5 minutes of collection, samples from 10 individuals were combined to ensure an adequate quantity for analysis.
Experimental design
Experiment 1: impact of varying TEB concentrations on chicken spermatozoa in vitro
This experiment aimed to investigate how different concentrations of TEB affect the quality and functional parameters of chicken spermatozoa in vitro. Pooled semen samples were divided into five equal subsamples, each containing 666 × 10⁶ sperm/ml, diluted with BPSE (Beltsville Poultry Semen Extender). The samples were then incubated at 36°C for 3 hours with the following TEB concentrations: (0 µM, 0.1 µM, 1 µM, 10 µM, 100 µM) (Heusinkveld et al., 2013). Our lowest TEB dose corresponded to approximately 30 ng/ml, which is comparable to the concentration of 29 ng/ml we obtained in a dietary experiment (not yet published) in the blood plasma after administering TEB through feeding. Semen quality was comprehensively analyzed after 1 and 3 hours of incubation. This analysis involved evaluating sperm motility parameters using the CASA system, and flow cytometry was employed to assess parameters such as membrane integrity, mitochondrial activity, acrosome integrity, chromatin structure, intracellular calcium levels, apoptosis markers (including early apoptosis and caspase activation), and lipid peroxidation. The experiment was repeated 8 times using the same roosters for consistency.
From these samples, after 3 hours of incubation, 100 µL aliquot was collected and centrifuged at 10,000 × g for 15 minutes at 4°C to separate the seminal plasma from the pellet. The separated components were then frozen at -82°C for further analyzes of malonyldialdehyde (MDA) concentration and total antioxidant capacity. MDA concentration was evaluated using Lipid Peroxidation assay kit accordingly manufacturer protocol and read at 532 nm by Microplate Reader (BioTek ELx800). The results are described using units per mL of seminal plasma (nM/mL) and pellet (nM/10 9 spz).
The Total Antioxidant Capacity (T-AOC) colorimetric assay kit was used for measurement of the cumulative antioxidant activity of all antioxidants presented in the seminal plasma. This assay is based on the ability of antioxidants to reduce a target compound, generating a colorimetric change that can be quantified spectrophotometrically. The intensity of the color is directly proportional to the total antioxidant capacity of the sample, allowing for an overall evaluation of its oxidative defense potential. The results are described using seminal plasma units (U/ml) measured using Microplate Reader (BioTek ELx800) absorbance at 520 nm.
Experiment 2: investigating calcium dynamics in chicken spermatozoa with calcium channel blockers
Based on the results of Experiment 1, which suggested that TEB may exhibit similar activity to calcium channel blockers, we conducted a subsequent study involving well-characterized representatives of this class. Pooled semen samples were divided into five subsamples, each containing 666 × 10⁶ sperm/ml diluted in BPSE, and treated as follows: Control (no blockers added), 20 µM nifedipine (L-type channel inhibitor, a dihydropyridine calcium channel blocker), 20 µM MRS 1845 (SOC inhibitor, a non-dihydropyridine calcium channel blocker), 60 nM SNX-325 (N-type channel inhibitor, a novel calcium channel blocker), 20 μM HC-056456 (a novel Ca²⁺ channel blocker, recognized as a distinctive compound that specifically targets the Catsper channel).
All blockers were dissolved in DMSO to achieve the required concentrations (Nguyen et al., 2016). Samples were incubated at 36°C for 3 hours, with assessments conducted at 1-hour and 3-hour intervals. Sperm motility parameters were evaluated using the CASA system, while flow cytometry was used to analyze membrane integrity, intracellular calcium levels, caspase activity, and lipid peroxidation.
Assessment of semen quality
To evaluate sperm motility, a computer-assisted semen analysis system (CASA Ceros II, Hamilton Thorne) was used. Semen samples were diluted in a 1:50 ratio with Dulbecco's Modified Eagle Medium (DMEM) containing low glucose to achieve a sperm concentration of approximately 50 × 10⁶/mL. A 3 µL aliquot of the diluted semen was placed in a pre-warmed analysis chamber (Leja, Nieuw-Vennep, Netherlands) at 36°C. For each sample, five random fields were examined. Parameters assessed included the percentage of motile sperm (MOT), progressively motile sperm (PROG), average path velocity (VAP), straight-line velocity (VSL), and curvilinear velocity (VCL).
Flow cytometry (Guava EasyCyte 5 cytometer, Merck Millipore) was employed to assess various sperm characteristics. Fluorescent probes were excited using a 488 nm argon ion laser, and data acquisition was managed with GuavaSoft 3.1.1 software. For each sample, 5000 events were analyzed, excluding non-sperm events based on scatter properties. All samples for cytometer analysis were diluted in BPSE to a concentration of 62 × 10⁶ per ml, unless otherwise specified in the instructions.
Plasma membrane integrity
Using the Live/Dead Sperm Viability kit, plasma membrane integrity (PMI) was assessed. Samples were stained with SYBR-14 (final concentration: 333 nM) and propidium iodide (PI; 40 µM) following the protocol by Partyka et al. (2010). SYBR-14 positive (green) and PI-negative sperm were considered to have intact membranes.
Mitochondrial activity
Mitochondrial membrane potential (HMMP) was assessed with JC-1 dye and PI. A stock solution of 3 mM JC-1 was prepared, and 500 µL of sperm suspension was stained with JC-1. Samples were incubated at 37°C in the dark for 20 minutes, followed by PI staining 3 minutes before analysis. Sperm showing only orange fluorescence were classified as high MMP (HMMP). Cells stained with PI were classified as non-viable.
Acrosome integrity
Acrosome integrity was determined using Alexa Fluor 488-conjugated lectin PNA. Samples were incubated for 5 minutes in a dark environment with a PNA solution (1 µg/mL), then analyzed with PI staining. This method distinguished live sperm with intact acrosomes (PI- PNA), live sperm with damaged acrosomes (PI- PNA+), dead sperm with intact acrosomes (PI+ PNA-), and dead sperm with damaged acrosomes (PI+ PNA+).
Chromatin status
Acridine orange (AO) staining was used to assess chromatin integrity. Normal double-stranded DNA exhibited green fluorescence, while denatured DNA emitted red fluorescence (DFI). Samples were diluted to a concentration of 1 × 10⁶ sperm/mL in BPSE and treated with an acid denaturation solution followed by AO staining as described by Partyka et al. (2010).
Calcium ion levels
Calcium levels were analyzed using Fluo 3 AM dye. Sperm diluted in BPSE were incubated with Fluo 3 AM (final concentration: 0.5 mM) at 37°C for 15 minutes. After incubation, samples were centrifuged, the supernatant removed, and PI was added before analysis to distinguish between live and dead cells with high and low calcium levels (Napierkowska, et al. 2024).
Apoptosis and membrane lipid disorder
Early apoptosis and membrane lipid disorder were evaluated using YO-PRO-1 (final concentration: 25 nM) and M540 (final concentration: 2.7 µM). Samples were incubated in the dark at room temperature for 10 minutes. Four populations were identified: live cells without apoptosis or lipid disorder (YO-PRO-1-/M540-), live cells with lipid disorder (YO-PRO-1-/M540+), apoptotic cells (YO-PRO-1+/M540+) and cells with apoptosis but no lipid disorder (YO-PRO-1+/M540-).
Caspase activity
Caspase activity was detected using the CellEvent™ Caspase 3/7 Green Ready Flow™ Reagent kit. Samples were prepared accordingly to the manufacturer's protocol. Both live and dead cells could be differentiated based on the activity of their caspases. This enabled the identification of four cell groups: live cells with active caspases, live cells with inactive caspases, dead cells with active caspases, and dead cells with inactive caspases.
Lipid peroxidation
Lipid peroxidation (LPO) in sperm cell membranes was assessed using the fluorescent probe C11-BODIPY581/591.This probe changes color from red (non-oxidized) to green (oxidized) upon exposure to reactive oxygen species. Samples were stained with the probe and incubated at 37°C for 30 minutes. Following centrifugation and re-suspension in BPSE, PI was added to identify viable sperm before cytometric analysis. In this experiment, we distinguish the following cell groups: live cells without lipid peroxidation (C11-BODIPY581/591red, PI -), live cells with lipid peroxidation (C11-BODIPY581/591 green, PI -), dead cells without lipid peroxidation (C11-BODIPY581/591 red, PI +) and dead cells with lipid peroxidation (C11-BODIPY581/591 green, PI +).
Statistical analysis
Statistical assessments were conducted using GraphPad Prism 10.4.0 Inc., 2024. For data analysis, tests were used depending on the distribution: one-way ANOVA for normal distribution and the Kruskal-Wallis test for non-normal distribution. Values were determined to be significant when * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001. The results are expressed as mean ± SEM.
Results
Experiment 1: impact of varying TEB concentrations on chicken spermatozoa in vitro
Sperm motility parameters analysis
Exposure to TEB concentrations of 0.1, 1, and 10 µM resulted in a significant decrease (P < 0.01) in the VAP parameter compared to the control group after 3 hours of incubation. No significant changes in this parameter were observed after 1 hour (Fig.1 A). Other sperm motility parameters, such as VCL, VSL, MOT, and PROG did not show significant differences between the groups after 1 hour and 3 hours of incubation (Fig.1 B,C,D,E).
Fig. 1.
The effect of different concentrations of TEB on chicken spermatozoa motility parameters after 1 h and 3 hrs of exposure. Path velocity (VAP) - A, curvilinear line velocity (VCL) -B, progressive velocity (VSL) - C, the percentage of motile sperm (MOT) - D, the percentage of progressively motile spermatozoa (PROG) - E. Different superscripts within the bars denote differences AB (P < 0.01).
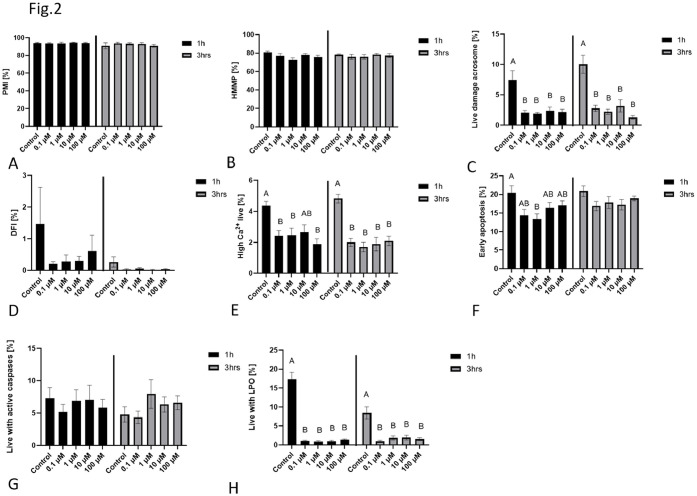
The effect of TEB on the integrity of the sperm plasma membrane was not significant whatever the tested concentrations after 1 and 3 hours of incubation (Fig. 2 A). HMMP remained stable at all tested concentrations and incubation times (Fig. 2 B). The percentage of live cells with damaged acrosomes significantly decreased (P < 0.01) at all tested concentrations (0.1 µM, 1 µM, 10 µM, and 100 µM) compared to the control after 1 hour of incubation. After 3 hours, acrosome damage remained lower at all TEB concentrations compared to the control (Fig. 2 C). DFI showed no significant changes across concentrations or incubation times (Fig. 2 D). TEB reduced the percentage of live sperm with high intracellular calcium (Ca²⁺) after 1 hour at 0.1 µM, 1 µM and 100 µM and after 3 hours of incubation at all concentrations (Fig. 2 E). Early apoptosis was significantly decreased at 1 µM, particularly after 1 hour of incubation. Higher concentrations did not further increase early apoptosis (Fig. 2 F). The activity of caspases remained unchanged (Fig. 2 G). Surprisingly, TEB effectively inhibited lipid peroxidation in live cells (P < 0.01) at all concentrations (Fig. 2 I).
Fig. 2.
The effect of different concentrations of TEB on chicken spermatozoa characteristics after 1 h and 3 hrs of exposure. Plasma membrane integrity (PMI) - A, high mitochondrial potential (HMMP) - B, live cells with damage acrosome – C, DNA fragmentation index (DFI) – D, high intercellular Ca²⁺ in live cells – E, early apoptosis – F, live cells with active caspases – G, live cells with LPO - H. Different superscripts within the bars denote differences AB (P < 0.01).
The results showed that the levels of MDA, a marker of lipid peroxidation, were significantly different between the control group and the TEB-treated groups. In both, seminal plasma and spermatozoa, the control group had exhibited the highest MDA levels. Treatment with TEB at all tested concentrations had resulted in a significant (P < 0.01) reduction in MDA levels (Fig. 3 A, B). In contrast, there had been no statistically significant differences in total antioxidant capacity (T-AOC) between the control group and the groups treated with TEB at any of the tested concentrations (P < 0.01) (Fig. 3 C).
Fig. 3.
The effect of different concentrations of TEB on malondialdehyde (MDA) concentration in seminal plasma (A), semen pellet (B) and total antioxidant capacity (T-OAC) in chicken spermatozoa (C) after 3 hrs of exposure. Different superscripts within the bars denote differences AB (P < 0.01).
Experiment 2: investigating calcium dynamics in chicken spermatozoa with calcium channel blockers
The analyzed motility parameters included VAP, VCL, VSL, MOT, and PROG were affected at different level by the treatments with specific blockers. For the parameter of VAP, the control group showed the highest values at both 1 hour and 3 hours, indicating optimal sperm velocity under normal conditions. All calcium channel blockers (SNX, MRS, Nifedipine, HC-056456) significantly reduced (P < 0.01) VAP, with this reduction being consistent across both time points (Fig. 4 A). A similar trend was observed for VCL, where the control group again exhibited the highest values. Treatments with calcium channel blockers significantly reduced VCL (P < 0.01) (Fig. 4 B).
Fig. 4.
The effect of different calcium channel blockers on motility parameters evaluated included path velocity (VAP) - A, curvilinear line velocity (VCL) -B, progressive velocity (VSL) - C, the percentage of motile sperm (MOT) - D, the percentage of progressively motile spermatozoa (PROG) – E of chicken spermatozoa after 1 h and 3 hrs of exposure. Different superscripts within the bars denote differences AB (P < 0.01).
For VSL, the control group demonstrated significantly higher values, while all blockers led to substantial reductions in straight-line velocity, mirroring the trends observed for VAP and VCL (Fig. 4 C). Similarly, sperm motility (MOT) was highest in the control group, while treatments with blockers, especially HC-056456, caused a noticeable decline in motility at both 1 hour and 3 hours (Fig. 4 D).
The PROG parameter revealed a type-dependent effect of a specific calcium channel blocker. The control group exhibited the highest progressive motility, while the blockers led to a significant (P < 0.01) decrease in PROG (Fig. 4 E).
The effects of calcium channel blockers on various semen parameters assessed by flow cytometry, measured after 1 hour and 3 hours, are presented in Fig. 5. PMI remained unaffected by the calcium channel blockers used, showing similar PMI percentages to the control group at both time points (Fig. 5 A).
Fig. 5.
The effect of different calcium channel blockers on flow cytometry characteristics after 1 h and 3 hrs of exposure. Plasma membrane integrity (PMI) - A, high intercellular Ca²⁺ in live cells – B, early apoptosis – C, live cells with active caspases – D, live cells with LPO - E. Different superscripts within the bars denote differences AB (P < 0.01).
Blockers such as SNX, MRS, Nifedipine, and HC-056456 caused a substantial reduction of live cells with high Ca²⁺, consistently at both 1 hour and 3 hours (Fig. 5 B). Early apoptosis levels remained stable across all groups, with no significant differences between treatments and the control at either time point (Fig. 5 C). The percentage of live sperm with active caspases was significantly lower (P < 0.05) in the groups treated with calcium channel blockers compared to the control group (Fig. 5 D). For live sperm with LPO, calcium channel blockers, particularly MRS, markedly reduced lipid peroxidation at both 1 hour and 3 hours (Fig. 5 F).
Discussion
These integrated results confirm the impact of various concentrations of TEB pesticides on chicken sperm function. Our goal was to study the various concentrations of TEB, one of the triazole fungicides that have been detected in the environment. We also compared our results with those of Serra et al. (2023b), who used the same concentrations but with shorter exposure times. Similar to their findings, exposure to TEB reduced VAP, but unlike their study, it did not significantly affect other mobility parameters. Analogous results were obtained in the experiment by Kabakci et al. (2021) conducted on bovine sperm, where motility parameters were also unaffected by TEB. As suggested by Jeyendran et al. (1984), reduced motility may be associated with an increased percentage of sperm with coiled tails, often linked to osmotic swelling. However, in our study, TEB did not affect sperm membrane integrity, which was consistent with the results of Kabakci et al. (2021) regarding the viability of Sertoli and Leydig cells as well as bovine spermatozoa. We also observed no significant changes in sperm DFI and HMMP. This suggests that although TEB affects sperm velocity, it does not cause significant structural damage to DNA or mitochondria at the concentrations tested. However, in vivo studies in mice demonstrated that triazoles induce mitochondrial dysfunction in female gametes. This led to ROS accumulation, apoptosis, and DNA damage in oocytes (Zhang et al., 2024).
Triazoles affect the integrity of sperm acrosomes (Pereira et al., 2019), which must undergo an acrosomal reaction necessary for fertilization. Acrosome damage can reduce the sperm's ability to penetrate the egg cell. In the present study, TEB inhibited the acrosomal damage process. It is possible that TEB interferes with the cell signaling or enzymatic processes responsible for triggering the acrosomal response, thus inhibiting the disruption of acrosome integrity. Calcium is an essential ion that regulates sperm movement, capacitation, and the acrosome reaction (AR), all of which are critical for successful fertilization (Finkelstein et al., 2020). As indicated by Chávez et al. (2018), the alkalization of the acrosome plays a pivotal role in triggering the release of Ca²⁺ from the acrosome, which subsequently initiates the acrosome reaction and the acrosome itself provides a storehouse for calcium ions in the cell.
The decreased calcium (Ca²⁺) levels in chicken spermatozoa at concentrations of 0.1 µM, 1 µM, 10 and 100 µM confirmed that TEB affects the calcium transport mechanisms across the sperm membrane. It is known that elevated Ca²⁺ levels can disrupt sperm motility and lead to premature apoptosis (Kruman and Mattson, 1999). As shown in the experiment by Heusinkveld et al. (2013), where five of the six azole fungicides induced nonspecific inhibition of voltage-gated calcium channels (VGCC), although with varying strength, suggesting a potential impact of TEB on these channels. As demonstrated in the study by Birch et al. (2022), TEB was one of the pesticides that, at a low nM dose, disrupted normal Ca2+ signaling in human sperm in vitro by CatSper channel. CatSper channels are considered crucial for the regulation of motility and acrosomal reaction in mammalian sperm (Darszon et al. 2011). However, some previous studies have reported CatSper genes as lost in birds during evolution (Cai and Clapham, 2008). In the present study we decided to block VOCCs (L and R type channels) located in the acrosome and central part of chicken sperm cells, as well as SOC in the acrosome, central part, nucleus, and tail (Nguyen et al., 2016). We also attempted to block CatSper channels. The use of a specific blocker for CatSper channels - HC-056456- blocked the influx of calcium ions into the cell, which may suggest the presence of similar channels in birds.
It is worth to highlight that calcium ions play an important role in sperm motility, regulating flagellar movement, metabolic pathways and energy production (Darszon et al., 1999, 2011) necessary to maintain other fertilizing ability (Chung et al. 2014). Building upon our previous findings, which demonstrated that certain pesticides, including imidacloprid (Napierkowska et al., 2024), can function as Ca²⁺ channel blockers, we aimed to investigate whether TEB exerts a comparable inhibitory effect. Blocking calcium channels disrupts calcium ion transport, leading to reduced sperm motility due to decreased calcium availability. The reduction in motility is most likely caused by impaired flagellar movement and ATPase activity, both of which depend on calcium (Chung et al. 2014). In the present study, blocking calcium channels did not have a significant impact on sperm viability, measured by PMI just as in the instance of TEB. Decreasing membrane permeability. Used herein, calcium channel blockers did not induce apoptosis either, indicating that sperm viability depends on factors other than calcium homeostasis. Our results once again confirmed that blocking Ca2+ channels with TEB did not lead to an increase in the acrosomal reaction, as shown by Nguyen et al. (2016). Remarkably, calcium channel blockers, especially MRS significantly reduced lipid peroxidation in chicken spermatozoa. Lipid peroxidation is a process that also depends on Ca²⁺ levels in cells. As shown by Al-Menhali et al. (2020), it is involved in the regulation of mitochondrial metabolism in skeletal muscles dependent on calcium. Our studies unequivocally demonstrated that blocking calcium channels led to a reduction in LPO. Similarly, in the case of another calcium channel blocker, VRP, used in studies on Russian sturgeon (Li et al., 2010), oxidative stress in sperm decreased, resulting in a reduction in LPO. Therefore, while calcium channel blockers disrupt sperm motility by interfering with calcium-dependent processes, they do not significantly affect sperm viability or induce apoptosis. This observation suggests that TEB's mechanism of action involves the modulation of calcium channels. By lowering calcium levels in the sperm cells and lowering sperm plasma membrane LPO, it displayed behavior indicative of channel blockade. Our studies showed also that TEB, by blocking calcium channels, can suppress early stages of apoptosis. As is known, excess calcium in mitochondria is a key factor in the apoptosis process (Kruman and Mattson, 1999). Reports clearly indicate that excessive calcium accumulation in cells or disruption of intracellular calcium distribution can lead to cellular toxicity and initiate both apoptosis and necrotic cell death (Orrenius et al., 2003). However, no increase in caspase activity was observed in our study. This outcome is likely explained by findings from Othmène et al. (2022), which suggest that TEB induces apoptosis via the mitochondrial pathway. Since TEB inhibited calcium influx in our experiment, mitochondrial integrity was preserved, preventing the activation of caspases. In contrast, calcium channel blockers influenced this process after just 1 hour of incubation. Notably, the ability of TEB to inhibit lipid peroxidation, despite its known potential to induce oxidative stress, may result from the reprogramming of sperm metabolism. This metabolic shift appears to limit cell membrane damage and progressively reduce ROS production over time. Alternatively, a second possible explanation could be the relatively low concentration of TEB used in our study, which may have been insufficient to trigger significant apoptotic pathways. As highlighted in the work of Aitken (1995, 2017), rising intracellular ROS levels initiate reactions that lead to LPO, during which nearly 60% of fatty acids in the membrane are lost. This process reduces membrane fluidity, increases nonspecific ion permeability, and inhibits membrane receptor and enzyme activity. These findings further support the conclusion that the concentrations tested in our study were not sufficient to elicit such effects. This aligns with the experiment conducted by Serra et al. (2023b), where only a 1 mM TEB dose after 24 minutes resulted in ROS formation. Therefore, our concentrations may have been too low to induce this effect.
To confirm the results obtained from flow cytometry, we evaluated the MDA level in the spermatozoa and seminal plasma after TEB treatment. The results further supported earlier findings that TEB reduces LPO. It is also worth noting that, at the same time when MDA decreased, total antioxidant capacity (T-AOC) remained unchanged. This may indicate that TEB, at the concentrations and exposure times used, does not significantly affect the overall antioxidant levels in sperm. Jannatifar et al. (2019) showed that there is an inverse correlation between MDA and T-OAC, so our concentrations may have been enough to lower MDA, but not enough to raise T-OAC.
Our study underscores the impact of low tebuconazole concentrations on chicken spermatozoa in modulating critical physiological processes in chicken spermatozoa, including motility, lipid peroxidation, apoptotic pathways, and calcium homeostasis. TEB functions as an inhibitor of calcium channels, effectively curtailing the intracellular influx of Ca²⁺ ions. This inhibition disrupts the formation of calcium-dependent membrane pores, thereby preserving membrane integrity, mitigating early apoptotic events, and reducing lipid peroxidation in chicken spermatozoa.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Ms. Renata Falczyńska from the company Tasomix for her valuable assistance.
This research was funded in whole by National Science Centre, Poland, grant number 2021/43/B/NZ9/01550.
The article is part of a PhD dissertation titled Mechanism of action of pesticides and their impact on roosters fertility, prepared during Doctoral School at the Wrocław University of Environmental and Life Sciences. The APC/BPC is financed by Wrocław University of Environmental and Life Sciences.
References
- Aitken R.J. Free radicals, lipid peroxidation and sperm function. Reprod. Fertil. Dev. 1995;7(4):659–668. doi: 10.1071/rd9950659. [DOI] [PubMed] [Google Scholar]
- Aitken R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017;84(10):1039–1052. doi: 10.1002/mrd.22871. [DOI] [PubMed] [Google Scholar]
- Al-Menhali A.S., Banu S., Angelova P.R., Barcaru A., Horvatovich P., Abramov A.Y., Jaganjac M. Lipid peroxidation is involved in calcium dependent upregulation of mitochondrial metabolism in skeletal muscle. Biochim. Biophys. Acta Gen. Subj. 2020;1864(3) doi: 10.1016/j.bbagen.2019.129487. [DOI] [PubMed] [Google Scholar]
- Baldi E., Luconi M., Bonaccorsi L., Krausz C., Forti G. Human sperm activation during capacitation and acrosome reaction: role of calcium, protein phosphorylation and lipid remodelling pathways. FBS. 1996;1:d189–d205. doi: 10.2741/a125. [DOI] [PubMed] [Google Scholar]
- Ben Othmène Y., Monceaux K., Belhadef A., Karoui A., Ben Salem I., Boussabbeh M., Abid-Essefi S., Lemaire C. Triazole fungicide tebuconazole induces apoptosis through ROS-mediated endoplasmic reticulum stress pathway. Environ. Toxicol. Pharmacol. 2022 doi: 10.1016/j.etap.2022.103919. [DOI] [PubMed] [Google Scholar]
- Birch M.R., Johansen M., Skakkebæk N.E., Andersson A.M., Rehfeld A. In vitro investigation of endocrine disrupting effects of pesticides on Ca2+-signaling in human sperm cells through actions on the sperm-specific and steroid-activated CatSper Ca2+-channel. Environ. Int. 2022;167 doi: 10.1016/j.envint.2022.107399. [DOI] [PubMed] [Google Scholar]
- Burrows W.H., Quinn J.P. The collection of spermatozoa from the domestic fowl and turkey. Poult. Sci. 1937;16(1):19–24. [Google Scholar]
- Cai X., Clapham D.E. Evolutionary genomics reveals lineage-specific gene loss and rapid evolution of a sperm-specific ion channel complex: CatSpers and CatSperb. PLoS One. 2008;3:e3569. doi: 10.1371/journal.pone.0003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J.E., Greim H., Kendall R.J., Segner H., Sharpe R.M., Van Der Kraak G. Human and ecological risk assessment of a crop protection chemical: a case study with the azole fungicide epoxiconazole. Crit. Rev. Toxicol. 2013;44(2):176–210. doi: 10.3109/10408444.2013.855163. [DOI] [PubMed] [Google Scholar]
- Chávez J.C., De la Vega-Beltrán, José O., Torres P., Nishigaki T., Treviño C.L., Darszon A. Acrosomal alkalization triggers Ca2+ release and acrosome reaction in mammalian spermatozoa. J. Cell. Physiol. 2018;233(6):4735–4747. doi: 10.1002/jcp.26262. [DOI] [PubMed] [Google Scholar]
- Chung J.J., Shim S.H., Everley R.A., Gygi S.P., Zhuang X., Clapham D.E. Structurally distinct Ca(2+) signaling domains of sperm flagella orchestrate tyrosine phosphorylation and motility. Cell. 2014;157(4):808–822. doi: 10.1016/j.cell.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darszon A., Labarca P., Nishigaki T., Espinosa F. Ion channels in sperm physiology. Physiol. Rev. 1999:481–510. doi: 10.1152/physrev.1999.79.2.481. [DOI] [PubMed] [Google Scholar]
- Darszon A., Nishigaki T., Beltran C., Treviño C.L. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 2011:1305–1355. doi: 10.1152/physrev.00028.2010. [DOI] [PubMed] [Google Scholar]
- Duzguner V., Erdogan S. Acute oxidant and inflammatory effects of imidacloprid on the mammalian central nervous system and liver in rats. Pestic. Biochem. Physiol. 2010;97(1):13–18. [Google Scholar]
- EFSA Scientific opinion of the panel on plant protection products and their residues (PPR Panel) on risk assessment for a selected group of pesticides from the triazole group to test possible methodologies to assess cumulative effects from exposure through food from these pesticides on human health. EFSA J. 2009;7(9):1167. [Google Scholar]
- Finkelstein M., Etkovitz N., Breitbart H. Ca2+ signaling in mammalian spermatozoa. Mol. Cell. Endocrinol. 2020;516:110953. doi: 10.1016/j.mce.2020.110953. [DOI] [PubMed] [Google Scholar]
- Heusinkveld H.J., Molendijk J., van den Berg M., Westerink R.H. Azole fungicides disturb intracellular Ca2+ in an additive manner in dopaminergic PC12 cells. Toxicol. Sci. 2013;134(2):374–381. doi: 10.1093/toxsci/kft119. [DOI] [PubMed] [Google Scholar]
- Huang T., Jiang H., Zhao Y., He J., Cheng H., Martyniuk C.J. A comprehensive review of 1,2,4-triazole fungicide toxicity in zebrafish (Danio rerio): a mitochondrial and metabolic perspective. Sci. Total Environ. 2022;809 doi: 10.1016/j.scitotenv.2021.151177. [DOI] [PubMed] [Google Scholar]
- Jacobsen P.R., Axelstad M., Boberg J., Isling L.K., Christiansen S., Mandrup K.R., Berthelsen L.O., Vinggaard A.M., Hass U. Persistent developmental toxicity in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reprod. Toxicol. 2012;34(2):237–250. doi: 10.1016/j.reprotox.2012.05.099. [DOI] [PubMed] [Google Scholar]
- Jannatifar R., Parivar K., Roodbari N.H., Nasr-Esfahani M.H. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod. Biol. Endocrinol. 2019;17(1):24. doi: 10.1186/s12958-019-0468-9. Feb 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyendran R.S., Van der Ven H.H., Perez-Pelaez M., Crabo B.G., Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J. Reprod. Fertil. 1984;70(1):219–228. doi: 10.1530/jrf.0.0700219. Jan. [DOI] [PubMed] [Google Scholar]
- Joshi S.C., Gulati N., Sharma B., Sharma P. Effects of tebuconazole (a fungicide) on reproduction of male rat. Int. J. Pharm. Res. Health. Sci. 2016;4:1489–1494. [Google Scholar]
- Kabakci R., Kaya A., Yigit A.A., Varisli O. Assessment of tebuconazole exposure on bovine testicular cells and epididymal spermatozoa. Acta. Vet. Hung. 2021;69(2):180–188. doi: 10.1556/004.2021.00023. [DOI] [PubMed] [Google Scholar]
- Kato T., Tanaka S., Ueda M., Kawase Y. Inhibition of sterol biosynthesis in Monilinia fructigena by the fungicide, S-1358. Agric. Biol. Chem. 1975;39(1):169–174. doi: 10.1080/00021369.1975.10861587. [DOI] [Google Scholar]
- Keber R., Rozman D., Horvat S. Sterols in spermatogenesis and sperm maturation. J. Lipid Res. 2013;54:20–33. doi: 10.1194/jlr.R032326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjærstad M.B., Taxvig C., Nellemann C., Vinggaard A.M., Andersen H.R. Endocrine disrupting effects in vitro of conazole antifungals used as pesticides and pharmaceuticals. Reprod. Toxicol. 2010:573–582. doi: 10.1016/j.reprotox.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Kruman I.I., Mattson M.P. Pivotal role of mitochondrial calcium uptake in neural cell apoptosis and necrosis. J. Neurochem. 1999;72(2):529–540. doi: 10.1046/j.1471-4159.1999.0720529.x. [DOI] [PubMed] [Google Scholar]
- Li Z.H., Li P., Rodina M., Randak T. Evaluating the function of calcium antagonist on the Cd-induced stress in sperm of Russian sturgeon, Acipenser gueldenstaedtii. Aquat. Toxicol. 2010;100(4):373–375. doi: 10.1016/j.aquatox.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Liu J., Tian Y.e., Ding Y.u., Heng D., Xu K., Liu W., Zhang C. Role of CYP51 in the regulation of T3 and FSH-induced steroidogenesis in female mice. Endocrinology. 2017;158(11):3974–3987. doi: 10.1210/en.2017-00249. [DOI] [PubMed] [Google Scholar]
- Napierkowska S., Froment P., Kowalczyk A., Pamuła J., Birger M., Niżański W., Partyka A. The neonicotinoid, imidacloprid, disrupt the chicken sperm quality through calcium efflux. Poult. Sci. 2024;103(9) doi: 10.1016/j.psj.2024.103959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.M., Duittoz A., Praud C., Combarnous Y., Blesbois E. Calcium channels in chicken sperm regulate motility and the acrosome reaction. FEBS J. 2016;283(10):1902–1920. doi: 10.1111/febs.13710. [DOI] [PubMed] [Google Scholar]
- Orrenius S., Zhivotovsky B., Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell. Biol. 2003;4(7):552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Partyka A., Nizański W., Łukaszewicz E. Evaluation of fresh and frozen-thawed fowl semen by flow cytometry. Theriogenology. 2010;74(6):1019–1027. doi: 10.1016/j.theriogenology.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Pereira V.R., Pereira D.R., de Melo Tavares Vieira K.C., Ribas V.P., Constantino C.J.L., Antunes P.A., Favareto A.P.A. Sperm quality of rats exposed to difenoconazole using classical parameters and surface-enhanced Raman scattering: classification performance by machine learning methods. ESPR. 2019;26(34):35253–35265. doi: 10.1007/s11356-019-06407-0. [DOI] [PubMed] [Google Scholar]
- Serra L., Bourdon G., Estienne A., Fréville M., Ramé C., Chevaleyre C., Didier P., Chahnamian M., Ganier P., Pinault F., Froment P., Dupont J. Triazole pesticides exposure impaired steroidogenesis associated to an increase in AHR and CAR expression in testis and altered sperm parameters in chicken. Toxicol. Rep. 2023;10:409–427. doi: 10.1016/j.toxrep.2023.03.005b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra L., Estienne A., Caria G., Ramé C., Jolivet C., Froger C., Henriot A., Amalric L., Guérif F., Froment P., Dupont J. In vitro exposure to triazoles used as fungicides impairs human granulosa cells steroidogenesis. Environ. Toxicol. Pharmacol. 2023;104 doi: 10.1016/j.etap.2023.104295a. [DOI] [PubMed] [Google Scholar]
- Strömstedt M., Rozman D., Waterman MR. The ubiquitously expressed human CYP51 encodes lanosterol 14 alpha-demethylase, a cytochrome P450 whose expression is regulated by oxysterols. Arch. Biochem. Biophys. 1996;329(1):73–81. doi: 10.1006/abbi.1996.0193. [DOI] [PubMed] [Google Scholar]
- Taxvig C., Hass U., Axelstad M., Dalgaard M., Boberg J., Andeasen H.R., Vinggaard AM. Endocrine-disrupting activities in vivo of the fungicides tebuconazole and epoxiconazole. Toxicol. Sci. 2007;100(2):464–473. doi: 10.1093/toxsci/kfm227. [DOI] [PubMed] [Google Scholar]
- van den Bossche H., Willemsens G., Cools W., Lauwers W.F., Le Jeune L. Biochemical effects of miconazole on fungi. II. Inhibition of ergosterol biosynthesis in Candida albicans. Chem. Biol Interact. 1978;21(1):59–78. doi: 10.1016/0009-2797(78)90068-6. [DOI] [PubMed] [Google Scholar]
- Witte T.S., Schäfer-Somi S. Involvement of cholesterol, calcium and progesterone in the induction of capacitation and acrosome reaction of mammalian spermatozoa. Anim. Reprod. Sci. 2007;102(3-4):181–193. doi: 10.1016/j.anireprosci.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang W., Zhang D., Zhang Y., Li Y., Fang F., Zhang Z., Zhang Y. Prothioconazole exposure disrupts oocyte maturation and fertilization by inducing mitochondrial dysfunction and apoptosis in mice. Free Radic. Biol. Med. 2024;213:274–284. doi: 10.1016/j.freeradbiomed.2024.01.027. [DOI] [PubMed] [Google Scholar]