Abstract
Carbon dioxide (CO2) generally exists as the main impurity in natural gas, whose main component is methane. The presence of CO2 reduces the energy content of natural gas and also causes the corrosion of pipelines. To prevent such problems, natural gas must contain a small concentration of CO2 (less than 2% by weight). Membrane technology is an attractive separation method that has been widely studied due to its advantages such as high efficiency, low operating costs, and low energy requirements. However, in the last decade, Mixed Matrix Membranes (MMMs) have attracted the attention of many researchers due to their suitable capabilities in separating polar from non-polar gases. In this research, a new MMMs was obtained by adding imidazole zeolite nanoparticle (ZIF-8) to the poly methyl pentene (PMP) polymer matrix. The polymer part of this membrane can provide high permeability and suitable mechanical and thermal stability. In addition, ZIF-8 particles enhance CO2 separation by offering high CO2 adsorption capacity and molecular sieving, improving selectivity. The gas permeability test was performed on pure and mixed matrix membranes at 30 ℃ and pressures of 2, 6 and 10 bar. In addition, the fabricated membranes were evaluated by FESEM, FTIR-ATR, BET, DMA and TGA tests. The results indicated that in the MMMs containing 30 wt% of nanoparticles in the polymer, the permeability of CO2 gas improved by more than 180% and reached about 278.95 barrer, compared to the pure polymer membrane at a pressure of 10 bar. Moreover, the selectivity of CO₂/CH₄ and CO₂/H₂ increased by 142% and 155%, respectively, primarily due to the preferential sorption of CO₂ over H₂ and CH₄ facilitated by ZIF-8 particles.
Keywords: Reverse selectivity, Mixed matrix membrane, Poly Methyl pentene, CO2 separation
Subject terms: Chemical engineering, Carbon capture and storage
Introduction
Separation processes have always been significantly important in the oil, gas and petrochemical industries, and various methods have been used for this purpose. Gases are used in large quantities in various industries, and this further specifies the necessity of purification and separation of gases from the gas mixture1. One of the most important processes in the gas industry is gas sweetening, in which CO2 and hydrogen sulfide (H2S) are separated from natural gas (CH4). The presence of CO2 gas in the pipeline along with natural gas causes pipe corrosion, formation of gas hydrates and loss of gas transmission energy2. Therefore, the separation of CO2 from natural gas in order to increase the calorific value of the gas and also to prevent the abovementioned issues, is a very important process in separation and transmission. Although the separation process is one of the most expensive steps of any chemical and petrochemical industry, a huge part of the cost of a product covers the separation process and the required equipment due to the high significance of this process. As a result, finding a cheaper and simpler separation method can be of great importance3. So far, various methods have been investigated for the removal and separation of CO2, including: absorption, cryogenic processes (with and without distillation), surface adsorption (thermal and pressure swing adsorptions) and membrane-based processes, from which the latter is a new and emerging technology compared to the rest of the mentioned methods4. In Membrane-based processes, gases are purified (separated from each other) with the aid of a permeable membrane, and different driving forces such as pressure, concentration and electric potential differences5. The advantages of this process over other methods is low energy consumption, suitable mass transfer rate, rapid membrane separation, no need for complex equipment and versatile installation and operating conditions6. Among different membranes, polymer membranes have attracted the attention of many researchers due to their low operating cost, variable and flexible design, cost-effective production along with easy construction7. However, regardless of all the advantages that polymer membranes could provide, they suffer from limitations and weaknesses in gas separation, the most important of which is the inverse relationship between the two parameters of selectivity and permeability. This means that high permeability and selectivity cannot be expected from polymers at the same time. Mixed matrix membranes (MMMs) are composed of a continuous polymer phase serving as the base and a dispersed phase, which may include zeolites, molecular sieves, or nano-sized particles. These membranes have been developed to enhance the gas separation performance of polymeric membranes8.
Among the types of gas separator membranes and especially polymer membranes, MMMs are particularly important because they have the ability to approach and overcome the Robson limit in gas separation9. Studies conducted on MMMs showed that their performance is not just adding the inherent characteristics of a particular phase. Various factors such as the correct selection of polymer and additives, the interface between particles and polymers, sedimentation and distribution of particles and particle size can affect the performance10. The type of the additive (dispersed phase) is known to be the most important factor in the development of MMMs. Therefore, researchers applied different nanoparticles, among which the best results were obtained with metal-organic frameworks. Metal-organic frameworks (MOFs) are a new emerging class of porous crystalline materials with one, two, or three-dimensional network, composed of metal ions or clusters with monodentate or polydentate organic linkers. These frameworks are highly compatible with polymer chains because they have various organic groups which can reduce the non-selective holes in the polymer-particle interface11. These MOFs have impressive merits including low density, flexibility and high specific surface (in energy storage technology, filtration and building production from lightweight materials), completely regular and uniform cavities, high porosity and chemical stability. The abovementioned properties are important factors for increasing the gas selectivity of MMMs and the reason for the superiority of this category of materials over zeolites and carbon materials12.
Zeolite imidazole frameworks-8 (ZIF-8) are a subset of MOFs, formed in a tetrahedral arrangement, where the metal clusters are connected by imidazole linkers. These particles can provide high surface area (due to the presence of transition metals such as cobalt), the ability to adjust the cavity, high crystallization, mechanical, thermal and chemical stability, versatility and functionalization for various application fields. Moreover, the high penetration and adsorption capability of these frameworks toward specified molecules enhances their flexibility during the adsorption process and therefore can be considered a good addition in the formation of MMMs13.
Another noteworthy point in the performance of MMMs is the type of the polymer. One of the most promising, commercially available polymers for membrane fabrication is poly methyl pentene polymer (PMP). PMP, also known as TPX, is an amorphous polymer based on acetylene (poly acetylene polymers have the highest permeability rate among known materials). In addition, this polymer is part of the polyolefin family, with glass transition temperature of 20 to 30 ℃ and crystallinity degree of 20 to 80%. PMP has a low density of 0.78 g per cubic centimeter, a fractional free volume of above 0.28 and the softening temperature of about 250 ℃. It is also the most permeable pure hydrocarbon-based polymer known, due to its very high free volume. Other notable properties include high chemical resistance, high thermal stability, and good permeability14.
The membranes generally used in the gas separation process are divided into two groups. The first group are conventional selective membranes in which the separation is based on the size and penetration rate of the particle inside the membrane. The second group are non-conventional reverse selective membranes with separation criterion based on the solubility of the particle inside the membrane.
In general, the solution-diffusion model is widely used for the gas transport mechanism in dense polymer membranes. According to this model, as shown in ‘’equation (1)’’ gas permeability (P) is equal to the product of permeability (D) and solubility (S).
The permeability unit is 1 Barrer = 1 × 10− 10 cm3 (STP) cm/cm2 s cmHg.
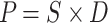 |
1 |
The ability of the membrane to separate different gases, for instance, components A and B, is equal to the permeability ratio of those two components, which is called selectivity (membrane separation factor). It is obtained according to the ‘’equation (2)’’:
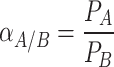 |
2 |
According to ‘’equation (3)’’ the ideal selectivity of two gases (A/B) is the product of solubility selectivity (SA/SB) and diffusivity selectivity (DA/DB). To obtain the optimal separation factor, the above two fractions must be complementary. Therefore, the permeability of the gas components could not only be attributed to the difference in the diffusion coefficient of the penetrant (diffusivity selectivity). The difference in chemical and physical interactions of these components with the polymer (solubility selectivity) should also be taken into consideration in the overall gas selectivity. The separation factor of a component in a gas mixture are determined by the balance between these two parameters15,16.
 |
3 |
Polymers with flexible chains possess high free volume (the space which is not occupied by polymer chains) while they have weak screening property based on size. In this regard, the passage of molecules through the membrane is facilitated and accelerated in larger polymers with increased free volume. Glassy polymers such as PTMSP, PMP, AF2400, PIM-1, etc. provide high permeability for larger condensable gases, due to the significant free volume17. In reverse selective membranes, the gases are separated based on their solubility in the membrane. Also, the features of the membranes have direct relationship with the free volume, therefore, it is possible to increase the free volume of the desired polymer by breaking polymer chains18. According to the free volume criteria, the reverse selectivity phenomenon can be justified with the help of this statistical mechanics model19, according to which, the diffusion coefficient of a component in the porous space is obtained from ‘’equation (4)’’:
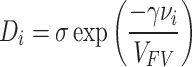 |
4 |
where, σ and γ (overlapping factor) are positive constants, vi is the minimum desired space (minimum empty volume) for component A to penetrate the polymer, Di is the penetration coefficient of the desired component, and VFV is the average free volume (average void volume) of the polymer. Adding ‘’equation (4)’’ to ‘’equation (3)’’ indicates that the selectivity of DA/DB is proportional to  . On the other hand, the value of vi is much smaller than VFV, so as the VFV increases, the
. On the other hand, the value of vi is much smaller than VFV, so as the VFV increases, the 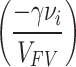 tends to zero and
tends to zero and 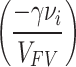 approaches one. As a result, the DA/DB ratio tends to one with the increase of the free volume of the polymer. Therefore, the improvement of selectivity due to the increase in the free volume of polymers could be validated based on the statistical mechanics model of Cohen and Turnball16.
approaches one. As a result, the DA/DB ratio tends to one with the increase of the free volume of the polymer. Therefore, the improvement of selectivity due to the increase in the free volume of polymers could be validated based on the statistical mechanics model of Cohen and Turnball16.
Accordingly, the aim of this work is to develop a new reverse selective membrane comprising high free volume polymer and MOF particles. PMP is used as a polymer phase of MMM and the synthesized ZIF-8 particles used as a dispersed phase within the membrane matrix. The combination of PMP and ZIF-8 enables reverse selectivity for CO₂ separation due to the high free volume of PMP, which facilitates solubility-driven transport rather than size-sieving, allowing CO₂ to permeate faster than smaller gases like H₂ or larger CH₄. ZIF-8, with its flexible pore apertures, exhibits a gate-opening effect that preferentially allows CO₂ diffusion while restricting larger or less condensable gases. This synergy between PMP’s intrinsic high permeability and ZIF-8’s selective adsorption and diffusion mechanisms results in enhanced CO₂ permeability, leading to reverse selectivity. A comprehensive characterization studies (i.e., FESEM, FTIR-ATR, BET, DMA and TGA) employed to study the physical properties of membranes. Moreover, a series of gas permeation tests including CO2/CH4 and CO2/H2 separation were designed over the different feed pressure to examine the gas separation performance of resulting MMMs.
Experimental
Materials
Poly methyl pentene polymer (PMP), in the form of milky pellets with low molecular weight was purchased from Sigma-Aldrich to be used as the polymer phase of the membrane. Carbon tetrachloride (CCl4) with a purity of 99.5% was purchased from Merck and used as a solvent of PMP. Likewise, ZIF-8 porous nanoparticles were synthesized and used as additives, using 2-methylimidazole, zinc nitrate and methanol purchased from Merck.
Synthesis of ZIF-8
In order to synthesize ZIF-8 nanoparticles, first, 2-methylimidazole (6.48 g) and zinc nitrate (2.933 g) were separately dissolved in 200 ml of methanol. The 2-methylimidazole solution was slowly poured into the zinc nitrate solution and stirred for 1 h. The final solution rested for 24 h and then centrifuged for 10 min at 10,000 rpm. Finally, after a sedimentation process of 12 h, the samples were washed three times by fresh methanol to remove unreacted precursors, organic molecules, or other residues. Finally the obtained clean solids were dried in an oven at a temperature of 65 ℃ for 12 h20.
Membrane preparation
Initially, the PMP polymer was placed in the oven at 60 ℃ for 6 h to remove any possible moisture. Then 5.7 gr of CCl4 solvent was added into a container with a gasketed lid and placed in an oil bath. The entire set was placed on a magnetic stirrer followed by the addition of 0.3 gr of the polymer in three separate steps (to prevent polymer aggregation). Next, the polymer solution samples were casted on a smooth, clean, glass plate using a film applicator with the thickness of 40 μm. The casted films were placed in an oven at a temperature of 50 ℃ for 6 h in order to completely evaporate the solvent. In case of the mixed matrix membrane, the manufacturing process was almost the same as the pure membrane. Only the dispersion of the additive nanoparticles in the CCl4 solvent was added to the overall process. In this regard, in the added step, ZIF-8 particles with different weight percentages were exposed to ultrasonic waves in the solvent for 10 min in order to avoid the non-uniform distribution of the particles. Next, the polymer was added step by step to the solution under stirring, same as the pure membrane method. Table 1 shows the percentage composition of each of the cast membranes21.
Table 1.
Solution composition of pure and MMMs membranes.
| Membrane code | Polymer (3 wt%) | Solvent (wt%) CCl4 | |
|---|---|---|---|
| PMP | ZIF-8 | ||
| M1 | 100 | 0 | 97 |
| M2 | 90 | 10 | 97 |
| M3 | 80 | 20 | 97 |
| M4 | 70 | 30 | 97 |
Membrane characterization
To determine the characteristic bonds in the prepared membranes, Fourier transform infrared spectrometry (FTIR-ATR) in the wave number range of 400–4500 cm− 1 and resolution of 4 cm− 1 was recorded on JASCO Model 300, Japan, system. Moreover, in order to monitor the surface and transverse structure of the obtained membranes, as well as the dispersion of nanoparticles in the polymer substrate, Field Emission Scanning Electron Microscopy (FESEM) was performed by MIRA3 TESCAN (Czech) device. In the sample preparation step, the pure and the mixed matrix membranes were broken in liquid nitrogen and then covered with gold under argon gas in order to achieve a suitable cross-sectional area and therefore proper resolution. The N2 adsorption-desorption test is based on measuring the volume of nitrogen gas absorbed and desorbed by the surface of the material, at a constant temperature of liquid nitrogen (77 K). Based on the measured absorption and desorption values of the material, this system can calculate the specific surface, the total volume of the holes and the average size of the holes of the material with the aid of Brunauer-Emmett-Teller (BET) theory. The device used in this test was the Belsorp mini II manufactured by the Japanese company Japan Bel. To investigate the degradation temperature and the effect of additives on the thermal behavior of mixed matrix membrane samples, TGA thermogravimetric analysis was conducted using TGA-50 device, Shimadzu. The nitrogen gas was applied at the heating rate of 10 ℃ per minute and the temperature range of 0 to 800 ℃. To investigate the glass transition temperature (Tg) and the mechanical properties of the membranes, mechanical dynamic analysis (DMA) was performed using the Pyris Diamond, USA device at a temperature range of 0 to 90 ℃ and a heating rate of 5 ℃/min. In order to measure the Young’s modulus of the samples, the temperature was increased from 25 to 200 ℃ with a repetition rate of 5 ℃/min. Tensile strength and elongation at break were calculated at a temperature of 25 ℃ and with an increased force of up to 20 N.
Gas permeability measurements
The system commonly used to identify the permeability of the constructed membranes is a constant volume/variable pressure system, which has the ability to measure permeability at different temperatures and pressures. According to this system, the desired membrane is placed between two steel discs (permeable cells) which are connected to each other, and the high-pressure gas hits the membrane from the feed side. The gas passing through the membrane enters a chamber of fixed volume, which increases the pressure inside the chamber with time. This pressure increase is accurately recorded by pressure sensors, and the amount of pressure variation is obtained in terms of time. Permeability of passing gas at 2, 6 and 10 bar pressure is calculated through ‘’equation (5)’’:
 |
5 |
where, P is the gas permeability (Barrer), V is chamber volume (cm3), L is the membrane thickness (cm), A is the surface area of the membrane (cm2), T is the test temperature (k), Po is gas feed pressure to the system (psia) and 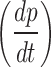 is the slope of the permeability gas pressure variation versus time.
is the slope of the permeability gas pressure variation versus time.
To determine the selectivity of CO₂ over CH₄ and H₂ in mixed matrix membranes (MMMs), the permeabilities of CO₂, CH₄, and H₂ are measured individually under identical conditions. Selectivity is then calculated by dividing the permeability of CO₂ by that of CH₄ to obtain CO₂/CH₄ selectivity, and by dividing the permeability of CO₂ by that of H₂ to obtain CO₂/H₂ reverse selectivity. The gas diffusion coefficient (D) (cm²/s) was calculated using the vacuum time lag method (Eq. 6):
 |
6 |
where θ is the gas time lag (s), determined by extrapolating the linear region of the pressure-time diagram.
By referencing Eq. 1 and incorporating the gas diffusion properties obtained from Eq. 6, the gas solubility (cm³(STP)/cm³·cmHg) can be calculated accordingly:
 |
7 |
Results and discussion
Fourier transform infrared spectroscopy – attenuated total reflectance (FTIR-ATR)
FTIR-ATR spectrum of additive particles, pure polymer membranes and mixed matrix membranes with different percentages of 10, 20 and 30% were recorded in the range of 400–4500 cm− 1 and the results depicted in Fig. 1. According to the Fig. 1, in the spectrum of ZIF-8 particles, the peak at 421 cm− 1 corresponds to the vibrational mode of Zn-N bond. The bands that appeared in the areas below 800 cm− 1 are often caused by the bending of the rings. A transition from 1143 to 1175 cm− 1 was observed, which can be related to the change of imidazole state to imidazolate. Moreover, the absence of any peak at 1850 cm− 1 confirms the formation of imidazolate22. The peak appearing at 1307 and 1421 cm− 1 represents C-N and C = N bond, respectively23. On the other hand, the absorbed signals at 1580 cm− 1 refer to the vibration of the rings. The stretching vibration of symmetric and asymmetric bonds of –CH2- also appeared in wave numbers of 2927 and 2957 cm− 1, respectively. Adsorption bands in the range of 2200 to 3300 cm− 1 are related to the vibration of formed hydrogen bonds24. In the PMP spectrum, methyl groups attached to the polymer chain with asymmetric stretching vibrations are observed at 2903 and 2952 cm− 1. In addition, the peaks at 500 to 900 cm− 1 are attributed to the symmetrical C-H bond. The stretching vibration of C-O-C is represented at 920 and 1167 cm− 1. MMMs also have the same bonds as pure polymer membranes. Due to the possible interactions between ZIF-8 and the polymer chains, the peaks characterizing these bonds are slightly shifted. An example of this displacement can be seen in the range of C-H and C-O-C bonds, which is also observed with some displacement in the MMMs, according to the figure25.
Fig. 1.
FTIR spectra of ZIF-8, PMP (M1), PMP/10 wt% ZIF-8 (M2), PMP/20 wt% ZIF-8 (M3), PMP/30 wt% ZIF-8 (M4).
Field emission scanning electron microscopy (FESEM)
In order to check the morphology of the synthesized membranes, the dispersion quality of nanoparticles and also to determine whether the constituents of the membranes are compatible with each other or not, the cross section of the membranes with different magnifications was tested by FESEM. The results are shown in Fig. 2. According to Fig. 2 (a), the pure polymer membrane has a uniform and dense structure with no defects. Figures 2 (b, c and d) illustrate the addition of ZIF-8 nanoparticles to the polymer matrix in which bright spots can be seen, representing the nanoparticles distributed in the dark space of the polymer. On the other hand, at higher weight percentages of additive particles, accumulation of particles was also observed. In addition, it is evident that the polymer/particle interface is of good quality and is almost free of any non-selective holes which can be attributed to the interaction between MOF organic groups and polymer chains. The cross-sectional images of the membranes with different weight percentages and in two different magnifications were also recorded. According to these images, as the weight% of ZIF-8 in the PMP network was raised, undesirable clusters of particles were observed in the images. The accumulation of particles results in the formation of non-selective cavities, which correspondingly reduces the performance of the synthesized membranes22,26,27.
Fig. 2.
The cross-sectional and surface images of (a) neat PMP, (b) PMP/ZIF-8 (10 wt%), (c) PMP/ZIF-8 (20 wt%), (d) PMP/ZIF-8 (30 wt%).
N2 adsorption-desorption analysis
In order to determine the structural characteristics of the synthesized ZIF-8 nanoparticles, the cell containing the desired sample was first placed in the liquid nitrogen tank. Then the amount of gas absorbed and desorbed by the material was calculated by gradually increasing and decreasing the nitrogen gas pressure at each stage. Finally, the Nitrogen adsorption and desorption diagram was drawn at constant temperature of 77 K. According to the obtained diagram (Fig. 3), the behavior of all particles follows isothermal type 1, representing the microporous structure28. In the desorption diagram, no residual is observed, especially in the relative pressure of more than 0.4, which indicates very low number of interporosity (mesopore) in the particle structure. The most important feature of this isotherm is that, at low pressures, a rapid increase in adsorption is seen, and at high pressures, the curve becomes an almost horizontal line. This isotherm is also called Langmuir curve. In this curve, a monolayer adsorption occurs at low pressure, which indicates a strong interaction between the gas and the adsorbent surface. The properties of ZIF-8 nanostructure synthesized with methanol solvent, such as pore diameter, pore volume, and BET surface area are compiled in Table 2. The properties of ZIF-8 nanostructure synthesized with methanol solvent, such as pore diameter, pore volume, and BET surface area are compiled in Table 2. The results obtained from BET analysis indicate that the synthesized ZIF-8 exhibits a high specific surface area, which is a characteristic feature of this material. Furthermore, the measured BET surface area values align well with those reported in previous studies, confirming the reliability and consistency of the synthesis method used in this work. This agreement suggests that the structural integrity and porosity of the synthesized ZIF-8 are comparable to those of ZIF-8 materials reported in the literature29,30.
Fig. 3.
N2 adsorption–desorption of ZIF-8 at 77 k.
Table 2.
BET analysis of ZIF-8 sample.
| Sample | SBET (m2g− 1) | Vtotal (cm3g− 1) | Dp (nm) |
|---|---|---|---|
| ZIF-8 | 1194.4 | 0.4751 | 1.5911 |
Thermogravimetric analysis (TGA)
The thermal behavior of the membranes prepared with different weight percentages of additive particles was studied with the aid of thermogravimetric decomposition test. TGA curves of ZIF-8 particles, PMP pure polymer and mixed matrix membranes are depicted in Fig. 4. The TGA diagram includes three parts: evaporation of moisture and solvent (in the temperature range of zero to 290 ℃), decomposition of polymer chains (in the range of 310 ℃) and decomposition of the organic part of the organic-metallic frameworks. Thermal degradation of PMP membrane chains started at a temperature of about 290 ℃. The degradation temperature (Td) in the PMP/ZIF-8 composite network membrane increased as the weight% of ZIF-8 particles was raised, finally reaching an approximate temperature of 355 ℃. The increased destruction temperature of the membrane can be due to the interaction between the particles and the polymer matrix, which strengthens and hardens the polymer chains31. On the other hand, the strong covalent bond between nanoparticles and polymer reinforces the polymer chains and therefore more energy is required to break these polymer chains. The precise quantification of ZIF-8 loading in MMMs was TGA analysis33. During the thermal decomposition process, the polymer matrix was completely degraded, leaving behind the inorganic ZIF-8 filler as a residual weight. The final residual weight% recorded in the TGA curves corresponds directly to the amount of ZIF-8 present in each membrane. By analyzing the residual mass at 800 °C, the calculated ZIF-8 loadings within the MMMs were determined to be 9.5 wt%, 19.7 wt%, and 28.9 wt%, corresponding to the M2, M3, and M4 membranes, respectively. These results confirm the effective incorporation of ZIF-8 into the polymer matrix and validate the reliability of the TGA method in accurately assessing the filler content in MMMs.
Fig. 4.
Thermal gravimetric analysis (TGA) curve of ZIF-8 and MMMs with loading of 10, 20 and 30 wt% of MOF.
Dynamic mechanical analysis (DMA)
In this analysis, an oscillating force is applied to the sample and the response is evaluated. Figure 5 indicates the changes of tanδ and modulus (É) in terms of temperature for pure and mixed matrix membranes. The maximum of the tanδ diagram (d tan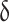 / dT = 0) represents the glass transition temperature (Tg) of the membranes. The peak located at 35 ℃ indicates the Tg of the PMP membrane, which is in accordance with previous reports32. Raising the weight% of ZIF-8 nanoparticles in the membrane increased the Tg values and the elastic modulus of the MMMs. This increase in Tg can be assigned to the confinement of polymer chains with ZIF-8 particles. The same is true for the elastic modulus, the presence of ZIF-8 nanoparticles strengthened the polymer chains. It should also be mentioned that at higher temperatures, the values of the elastic modulus of the MMMs reduced33.
/ dT = 0) represents the glass transition temperature (Tg) of the membranes. The peak located at 35 ℃ indicates the Tg of the PMP membrane, which is in accordance with previous reports32. Raising the weight% of ZIF-8 nanoparticles in the membrane increased the Tg values and the elastic modulus of the MMMs. This increase in Tg can be assigned to the confinement of polymer chains with ZIF-8 particles. The same is true for the elastic modulus, the presence of ZIF-8 nanoparticles strengthened the polymer chains. It should also be mentioned that at higher temperatures, the values of the elastic modulus of the MMMs reduced33.
Fig. 5.
(a) tan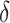 curve, (b) storage modulus versus temperature for PMP, PMP/10 wt% MOF, PMP/20 wt% MOF and PMP/30 wt% MOF.
curve, (b) storage modulus versus temperature for PMP, PMP/10 wt% MOF, PMP/20 wt% MOF and PMP/30 wt% MOF.
The mechanical properties of PMP membranes and composite networks containing 10, 20 and 30 wt% of ZIF-8 nanoparticles are summarized in Table 3. A reduction in the tensile strength and elongation at break was observed as the weight% of nanoparticles in the membrane was increased. The reduction in tensile strength with increasing ZIF-8 particle content in MMMs can be explained by several factors. Higher ZIF-8 content can increase brittleness by restricting the mobility of PMP chains, making the membrane more prone to fracture under stress. Furthermore, non-uniform dispersion of ZIF-8 particles at high loadings can cause agglomeration, leading to stress concentration points that reduce tensile strength. Contrary to tensile strength and elongation, Young’s modulus increased in mixed matrix membranes. The increase in Young’s modulus in mixed matrix membranes (MMMs), despite a reduction in tensile strength and elongation, can be explained by the incorporation of rigid filler particles (such as ZIF-8) into the polymer matrix. These particles act as a reinforcing phase, restricting the mobility of the polymer chains and making the membrane stiffer. As a result, the membrane resists deformation more strongly under stress, which leads to an increase in Young’s modulus, a measure of stiffness.
Table 3.
Mechanical properties of ZIF-8 filled PMP membranes at different loadings.
| Membrane | Tensile strength (MPa) | Elongation at break (%) | Young modulus (GPa) |
|---|---|---|---|
| PMP | 137 ± 2 | 121 ± 5 | 4.19 ± 0.2 |
| PMP/10 wt% ZIF | 126 ± 3 | 116 ± 2 | 4.72 ± 0.2 |
| PMP/20 wt% ZIF | 119 ± 2 | 108 ± 3 | 5.06 ± 0.1 |
| PMP/30 wt% ZIF | 107 ± 4 | 99 ± 3 | 5.44 ± 0.3 |
Gas permeation results
The effect of nanoparticles on permeability and selectivity
The permeability values of CO2, CH4 and H2 gases and the selectivity of CO2/CH4 and CO2/H2 in pure polymer membranes and mixed matrix membranes, at 30 ℃ and 2 bar pressure, are given in Table 4.
Table 4.
Effect of ZIF-8 content on PMP/ZIF-8 membranes at 2 bar.
| Membrane Code | P (CO2) | P (CH4) | P (H2) | CO2/CH4 | CO2/H2 |
|---|---|---|---|---|---|
| M1 | 99.13 | 7.38 | 8.52 | 13.43 | 11.63 |
| M2 | 139.24 | 7.61 | 8.84 | 18.29 | 15.75 |
| M3 | 194.32 | 7.95 | 9.01 | 24.44 | 21.56 |
| M4 | 259.81 | 8.54 | 9.24 | 30.42 | 28.11 |
According to the data, for all the MMMs containing ZIF-8, the permeability values of CO2 were significantly higher than the corresponding values of CH4 and H2, the values increased as the weight% of the additive was increased. For instance, in the PMP membrane, the permeability of CO2, CH4 and H2 gases were 99.13, 7.38 and 8.52 Barrer, respectively, while the permeability values in the mixed matrix membrane containing 30 wt% of ZIF-8 particles enhanced to 259.81, 8.54 and 9.24 Barrer, respectively. Higher condensability and lower kinetic radius of CO2 gas (the kinetic radius of CO2 is equal to 0.33 nm and CH4 is equal to 0.38 nm), compared to CH4, along with the mutual effects between PMP polymer chains and CO2 justifies the increased permeability toward CO2. Likewise, this assertion is also true for H2 gas (despite the kinetic radius of 0.29 nm, which is lower than CO2). As illustrated in Fig. 6, increasing the weight% of ZIF-8 particles in the polymer network of the membrane improved the permeability of CO2, CH4 and H2 gases. The obtained permeability of CO2 gas is much higher in comparison to CH4 and H2 gases. Two factors are responsible for the improvement of CO2 permeability in PMP/ZIF-8 mixed matrix membrane. First, the presence of ZIF-8 nanoparticles in the mixed matrix membrane increases the free volume of the membrane which facilitates higher condensability of CO2. Second, the interaction between CO2 gas quadrupoles with imidazolate linker in ZIF-8 nanoparticles leads to improved CO2 permeability. On the other hand, unlike CO2, the presence of ZIF-8 particles in the polymer matrix had much smaller effect on improving the permeability of CH4 and H2. This minor increase can be related to the weak absorption of these gases by ZIF-8 and the increased penetration in the polymer membrane due to the increased free volume of the polymer34,35.
Fig. 6.
The effect of ZIF-8 content and pressure changes on (a) CO2 permeability, (b) CH4 permeability, (c) H2 permeability, (d) CO2/CH4 selectivity, and (e) CO2/H2 selectivity.
The effect of nanoparticles on permeability and solubility coefficients
Figure 7 depicts the values of diffusion coefficient and solubility of CO2, CH4 and H2 in PMP polymer and mixed matrix membranes. Gas penetration and solubility in the membrane are the two influential factors in gas permeability. The diffusion coefficient values of all three gases increase in exchange for increasing the weight% of ZIF-8 nanoparticles, which can be due to the escalated free volume of the polymer. It should be taken into consideration that the presence of ZIF-8 nanoparticles elongates the gas penetration path and therefore can diminish the permeability. In general, the explanation of how the gases penetrate in the MMMs is somehow complicated and it is not possible to provide a conclusive verification of their behavior. In addition, the penetration of a gas in the membrane is a function of its molecular size, therefore, CO2 gas passes through the space in the membrane more easily due to its smaller molecular size, in comparison to the other two gases. Gas solubility values in MMMs were higher than the corresponding values in PMP polymer membrane. The solubility coefficient of CO2 in the PMP/ZIF-8 membrane increased from 15.96 to 30.32 (about 90%), while a very small improvement was observed in the solubility coefficient of CH4 and H2. The significant increase in CO2 solubility in the presence of ZIF-8 particles can be attributed to the high tendency of this nanoparticle to absorb CO2 and the greater condensability of CO2 in the polymer matrix. The reason for this high affinity is the interaction between stable CO2 quadrupoles with the imidazolate linker in ZIF-8. The lack of interaction between nanoparticles with CH4 and H2 gases, along with the very low condensability of these two gases, has led to an insignificant increase in their solubility36,37.
Fig. 7.
the effect of filler loading on CO2, CH4 and H2. Diffusion and solubility coefficients.
The values of diffusion selectivity (DCO2/DCH4), (DCO2/DH2) and dissolution selectivity (SCO2/SCH4), (SCO2/SH2) are shown in Table 5. The dissolution selectivity increased with the addition of ZIF-8 nanoparticles, which has the main effect on the overall selectivity. The MOF in the polymer matrix separates the desired gases based on selective absorption, not based on screening properties, therefore, the dissolution selectivity value of SCO2/SCH4=11.09 and SCO2/SH2=15.60 in PMP membrane increased to 20.05 and 26.29 in PMP/ZIF-8 membranes, respectively. The permeation of CH4 and H2 gases is reduced due to the hardening of PMP polymer chains and the lengthening of the permeation path (due to the presence of ZIF-8 nanoparticles), and as a result, permeation selectivity is improved from DCO2/DCH4=1.21 and DCO2/DH2=0.72 in PMP polymer membrane to 1.26 and 0.87 in PMP/ZIF-8 membranes, respectively. On the other hand, one of the characteristics of reverse selectivity membranes is the separation of gases based on solubility, and the contribution of permeation selectivity in gas separation is decreased. In the studied MMMs, the permeation selectivity values are close to one and its contribution to CO2/CH4 and CO2/H2 selectivity dwindled. According to the obtained results and the abovementioned explanations, it can be concluded that the PMP/ZIF-8 membrane possess better reverse selectivity properties.
Table 5.
Effect of ZIF-8 content on PMP/ZIF-8 membranes at 2 bar.
| Membrane Code | DCO2/DCH4 | DCO2/DH2 | SCO2/SCH4 | SCO2/SH2 |
|---|---|---|---|---|
| M1 | 1.21 | 0.72 | 11.09 | 15.60 |
| M2 | 1.26 | 0.76 | 12.42 | 17.68 |
| M3 | 1.27 | 0.81 | 15.78 | 21.61 |
| M4 | 1.26 | 0.87 | 20.05 | 26.29 |
Effect of pressure on the performance of MMMs in separation of CO2/CH4 and CO2/H2
Figure 8 indicates the effect of feed pressure on the permeability of CO2, CH4 and H2 gases, as well as the selectivity of CO2/CH4 and CO2/H2 in polymer and mixed matrix membranes with varied weight percentages and at a temperature of 30 ℃. In the PMP polymer membrane, with the increase in pressure, the permeability of gases encountered a slight decrease; the permeability of CO2 changed from 101.67 Barrer at a pressure of 2 bar to 99.13 Barrer at a pressure of 6 bar. The permeability of CH4 and H2 at a pressure of 2 bar decreased from 7.24 to 8.95 Barrer to 7.38 and 8.52 Barrer at 6 bar pressure, respectively. Increasing the pressure on the feed side densifies the polymer chains and as a result reduces the penetration of gases in the polymer network of the membrane, which can also result in a reduction in permeability. According to the figure, the permeability of CO2 in the MMMs enhanced with the increase in the weight% of nanoparticles at higher pressures. For instance, the permeability of CO2 in the membrane containing 30 wt% of ZIF-8 particles increased from 234.41 Barrer at 2 bar pressure to 259.81 Barrer at 6 bar pressure. This increasing trend in MMMs is only evident in CO2 gas and is not seen in other gases. One of the main reasons for this variation in permeability is the increase in the concentration of condensable gases such as CO2, which causes the softening phenomenon in the membrane. Figure 8 indicates the changes in selectivity of CO2/CH4 and CO2/H2 in pure PMP membrane and MMMs with different weight percentages, with respect to feed pressure. The increase in feed pressure improved the selectivity of CO2/CH4 and CO2/H2 in MMMs. The adsorption through MOF particles along with the condensation are considered the main factors of gas permeability variation in MMMs at higher pressures. Factors affecting the improvement of the selectivity of CO2/CH4 and CO2/H2 in membranes containing ZIF-8 particles, it can be mentioned the reduction of permeability of CH4 and H2 due to the hardening of polymer chains, especially in the polymer/particle joint phase38.
Fig. 8.
The effect of pressure on permeability of (a) CO2, (b) CH4, (c) H2 and selectivity of (d) CO2/CH4, (e) CO2/H2.
According to Fig. 9, the permeability coefficient for pure membranes and mixed matrix membranes slightly decreased with increasing pressure. At higher pressures, the membrane becomes denser and the free volume of the membrane decreases, which can lead to a reduction in the gas permeability coefficient39. For this reason, the diffusion coefficient of these gases did not have a noticeable dependence on the pressure change. Another factor which is affected by pressure variation is the solubility coefficient of gases. In general, increasing the gas pressure will increase the gas concentration upstream of the membrane and can have a more favorable effect on the gas solubility coefficient. CO2 gas has higher compressibility compared to CH4 and H2 gases, this positive feature along with the favorable interactions of CO2 with the prepared membranes significantly enhanced the solubility coefficient of this gas with increasing pressure. On the other hand, the pressure increase had no significant effect on the solubility coefficient of CH4 and H2 gases due to low compressibility and lack of proper interaction with the constructed membranes40. Figures 10 and 11 show the performance of pure membranes and mixed matrix membranes in the separation of CO2/CH4 and CO2/H2 compared to Robson’s limit. According to the figure, better performance of the membrane is observed in the weight% and also at higher pressures, compared to Robson’s limit.
Fig. 9.
The effect of pressure on diffusion coefficient of (a) CO2, (b) CH4, (c) H2 and solubility coefficient of (d) CO2, (e) CH4, (f) H2.
Fig. 10.
Comparison of CO2/CH4 separation performances of pure PMP, PMP/ZIF-8 with Robeson present and prior bound.
Fig. 11.
Comparison of CO2/H2 separation performances of pure PMP, PMP/ZIF-8 with Robeson present and prior bound.
Mixed gas tests
The mixed gas permeation properties of the PMP/ZIF-8 mixed matrix membranes (MMMs) were investigated using equimolar (50:50 v/v) gas mixtures of CO2/CH4 and CO2/H2. The experiments were conducted at a pressure of 6 bar and a temperature of 30 °C. After passing the gas mixture through the membrane, the impermeable flow was removed, and the composition of the permeable gas flow was determined using gas chromatography (GC). The permeability and selectivity results for the mixed gas conditions are presented in Table 6.
Table 6.
Mixed gas permeability and selectivity data at 6 bar and 30 °C compared to pure gas tests.
| Pure gas tests | Mixed gas tests | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Permeability (barrer) | Ideal selectivity | Permeability (barrer) | Real selectivity | ||||||
| CO2 | CH4 | H2 | CO2/CH4 | CO2/H2 | CO2 | CH4 | H2 | CO2/CH4 | CO2/H2 |
| PMP/10 wt% ZIF-8 | |||||||||
| 139.24 | 7.61 | 8.84 | 18.29 | 15.75 | 128.35 | 7.56 | 8.69 | 16.97 | 14.76 |
| PMP/20 wt% ZIF-8 | |||||||||
| 194.32 | 7.95 | 9.01 | 24.44 | 21.56 | 185.26 | 7.84 | 8.84 | 23.63 | 20.95 |
| PMP/30 wt% ZIF-8 | |||||||||
| 259.81 | 8.54 | 9.24 | 30.42 | 28.11 | 248.38 | 8.36 | 9.11 | 29.71 | 27.26 |
A comparison of the mixed gas permeability results with the pure gas data indicates a decline in CO2 permeability and selectivity in all MMMs. This reduction is attributed to the competitive sorption and transport effects that arise in the presence of CH4 and H2 in the mixture.
This reduction occurs due to the presence of CH4 and H2 in the mixture, which inhibits CO2 transport through the membrane. The decrease in permeability is more pronounced in MMMs with higher ZIF-8 loading, suggesting that ZIF-8 particles have a role in CO2 adsorption competition. The ideal CO2/CH4 selectivity values for PMP/10, 20, and 30 wt% ZIF-8 were 18.29, 24.44, and 30.42, respectively. Under mixed gas conditions, these values reduced to 16.97, 23.63, and 29.71, respectively. Similarly, the ideal CO2/H2 selectivity decreased from 15.75, 21.56, and 28.11 in pure gas conditions to 14.76, 20.95, and 27.26, respectively, in mixed gas. The decrease in gas permeability and CO2 selectivity over CH4 and H2 can be attributed to the following series of phenomena:
The presence of CH4 in the gas mixture reduces CO2 adsorption on the ZIF-8 particles. Since ZIF-8 has an affinity for CO2, the simultaneous presence of CH4 reduces the effective number of adsorption sites available for CO2.
The presence of CH4 in the gas mixture reduces CO2 adsorption on the ZIF-8 particles. Since ZIF-8 has an affinity for CO2, the simultaneous presence of CH4 reduces the effective number of adsorption sites available for CO2.
The presence of CH4 inside the membrane matrix, especially within the free volume, inhibits the condensation of CO2. This reduces the solubility of CO2, thereby lowering its overall permeability.
In mixed gas conditions, CH4 and H2 molecules occupy free volume spaces within PMP, disrupting the CO2 transport pathways.
Gas separation performance
The gas separation results of the prepared membranes were compared with the results of various articles about MMMs with Robseon’s upper limit and the results are shown in Figs. 12 and 13. It was revealed that not only the gas separation properties of the fabricated MMMs in this research was better than most of the other reported MMMs, but also exceeded the upper limit of Robson, which confirms the optimal performance of the prepared membranes. In addition, outstanding results were obtained, even at low amount of nanoparticles, which can reduce the cost of membrane construction.
Fig. 12.
A comparison between the results of the present study and other MMMs in CO2/CH4 separation.
Fig. 13.
A comparison between the results of the present work and other MMMs in CO2/H2 separation.
Conclusions
In this research, new MMMs were fabricated using ZIF-8 nanoparticles and PMP polymer. In order to evaluate the gas separation performance of the prepared membranes, a gas permeability test was conducted at a constant temperature of 30 ℃ and pressures of 2, 6 and 10 bar, using a constant volume-variable pressure device. In addition, various tests such as FESEM, FTIR-ATR, BET, TGA and DMTA were applied to investigate and analyze the structure of the membranes. The obtained results demonstrated that the nanoparticles had very good compatibility with the PMP polymer, with uniform distribution over the polymer bed without any accumulation. The polymer chains surrounded the nanoparticles without any holes or defects. Based on gas permeability results, the most appropriate performance was observed with MMMs containing 30% by weight of nanoparticles, in which CO2 gas permeability improved by more than 180% compared to the pure polymer membrane at a pressure of 10 bar and reached about 278.95 Barrer. Meanwhile, the selectivity of CO2/CH4 and CO2/H2 improved by 142% and 155% respectively at 10 bar pressure and reached 33.36 and 30.35.
Acknowledgements
The authors acknowledge Babol Noshirvani University of Technology for financial support of this project (Grant NO. BNUT/393054/2021).
Author contributions
Authors’ contributionsThe authors’ contributions are shown as below: Behnam Majid-Nateri: Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Visualization. Reza Abedini: Conceptualization, Methodology, Validation, Formal analysis, Resources, Data curation, Writing – review & editing, Supervision, Project administration, Funding acquisition. Alireza Amiri: Validation, Formal analysis, Investigation, Writing – original draft, Visualization.
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nematollahi, M. H. et al. Recent progress on pebax-based thin film nanocomposite membranes for CO2 capture: the state of the art and future outlooks. Energy Fuels. 36(20), 12367–12428 (2022). [Google Scholar]
- 2.Ranjbar, F. et al. The experimental/theoretical study over the effect of using the POP-NH. Nanostruct. Into Membrane Selective Layer. CO2 Permeability Selectivity Chem. Eng. Res. Des.187, 184–195 (2022). [Google Scholar]
- 3.Zhu, T. et al. Preparation of poly (ether-block‐amide)/poly (amide‐co‐poly (propylene glycol)) random copolymer blend membranes for CO2/N2 separation. Polym. Eng. Sci.59, 14–23 (2019). [Google Scholar]
- 4.Tajdini, Z. & Feijani, E. A. Highly promoted CO2 separation of poly (ether-block-amide) based mixed matrix membranes using MOFs@ aminoclay architectures as fillers. Sci. Rep.14(1), 29082 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassanzadeh, H., Abedini, R. & Ghorbani, M. CO2 separation over N2 and CH4 light gases in sorbitol-modified Poly (ether-block-amide)(pebax 2533) membrane. Ind. Eng. Chem. Res.61(36), 13669–13682 (2022). [Google Scholar]
- 6.Jumaah, A. F. & Abedini, R. Fabrication and performance evaluation of mixed matrix membrane comprising Pebax and graphene hydroxyl in olefin/paraffin separation. Polyolefins J.12, 31–44 (2025).
- 7.Zhu, T. et al. Random and block copolymer membranes based on flexible etheric-aliphatic soft segments designed for CO2/CH4 separation. J. Nat. Gas Sci. Eng.54, 92–101 (2018). [Google Scholar]
- 8.Doosti, M. & Abedini, R. Polyethyleneglycol-modified cellulose acetate membrane for efficient olefin/paraffin separation. Energy Fuels. 36(17), 10082–10095 (2022). [Google Scholar]
- 9.Noorani, N., Mehrdad, A. & Shamszadeh, P. PVC-based mixed-matrix membranes based on IL@ AC/ NH2-MIL-101 nanocomposites for improved CO2 separation performance. Sci. Rep.14(1), 23843 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kheirtalab, M., Abedini, R. & Ghorbani, M. Pebax/poly (vinyl alcohol) mixed matrix membrane incorporated by amine-functionalized graphene oxide for CO2 separation. J. Polym. Sci.62(3), 517–535 (2024). [Google Scholar]
- 11.Zhang, D. S. et al. Fluorous metal-organic frameworks with enhanced stability and high H2/CO2 storage capacities. Sci. Rep.3(1), 3312 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behera, A. Metal-organic frameworks. In Advanced Materials. P 637–666 (Springer, 2022).
- 13.Khoshhal Salestan, S., Rahimpour, A. & Abedini, R. Experimental and theoretical studies of biopolymers on the efficient CO2/CH4 separation of thin-film Pebax® 1657 membrane. Chem. Eng. Processing-Process Intensif.163, 108366 (2021). [Google Scholar]
- 14.Abdollahi, S. A. & Ranjbar, S. F. Modeling the CO2 separation capability of Poly (4-methyl-1-pentane) membrane modified with different nanoparticles by artificial neural networks. Sci. Rep.13(1), 8812 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao, Y. et al. The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas—A review. Prog. Polym. Sci.34(6), 561–580 (2009). [Google Scholar]
- 16.Merkel, T. et al. Ultrapermeable, reverse-selective nanocomposite membranes. Science. 296(5567), 519–522 (2002). [DOI] [PubMed]
- 17.Golubev, G. S. et al. High free volume polymers for pervaporation. Curr. Opin. Chem. Eng.36, 100788 (2022). [Google Scholar]
- 18.Lau, C. H. et al. Reverse-selective polymeric membranes for gas separations. Prog. Polym. Sci.38(5), 740–766 (2013). [Google Scholar]
- 19.Cohen, M. H. & Turnbull, D. Molecular transport in liquids and glasses. J. Chem. Phys.31(5), 1164–1169 (1959). [Google Scholar]
- 20.Sun, L. et al. N self-doped ZnO derived from microwave hydrothermal synthesized zeolitic imidazolate framework-8 toward enhanced photocatalytic degradation of methylene blue. J. Colloid Interface Sci.565, 142–155 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Abedini, R., Mosayebi, A. & Mokhtari, M. Improved CO2 separation of Azide cross-linked PMP mixed matrix membrane embedded by nano-CuBTC metal organic framework. Process Saf. Environ. Prot.114, 229–239 (2018). [Google Scholar]
- 22.Amedi, H. R. & Aghajani, M. Functionalized Zeolitic-Imidazole Frameworks-8 based Poly (ether-b-amide) nanocomposite membrane for CO2/CH4 gas separation: synthesis and characterization. Iran. J. Polym. Sci. Technol.31, 3–14 (2018). [Google Scholar]
- 23.Zhu, T. et al. ZIF-8@ GO composites incorporated polydimethylsiloxane membrane with prominent separation performance for ethanol recovery. J. Membr. Sci.598, 117681 (2020). [Google Scholar]
- 24.Nematollahi, M. H., Babaei, S. & Abedini, R. CO2 separation over light gases for nano-composite membrane comprising modified polyurethane with SiO2 nanoparticles. Korean J. Chem. Eng.36, 763–779 (2019). [Google Scholar]
- 25.Shirzad Kebria, M. R. et al. Experimental and theoretical investigation of thin ZIF-8/chitosan coated layer on air gap membrane distillation performance of PVDF membrane. Desalination. 450(15), 21–32. (2019).
- 26.Nobakht, D. & Abedini, R. A new ternary Pebax® 1657/maltitol/ZIF-8 mixed matrix membrane for efficient CO2 separation. Process Saf. Environ. Prot.170, 709-719 (2023).
- 27.Mansourpanah, Y. & Bagri, F. Improvement of the performance of PDMS top layer of mixed matrix membrane incorporated with treated ZIF-8 for gas separation. J. Membrane Sci. Res.7(2), 111–117 (2021). [Google Scholar]
- 28.Fakoori, M. et al. Effect of Cu-MOFs incorporation on gas separation of Pebax thin film nanocomposite (TFN) membrane. Korean J. Chem. Eng.38, 121–128 (2021). [Google Scholar]
- 29.Zhang, H., Zhao, M. & Lin, Y. Stability of ZIF-8 in water under ambient conditions. Microporous Mesoporous Mater.279, 201–210 (2019). [Google Scholar]
- 30.Lee, Y. R. et al. ZIF-8: A comparison of synthesis methods. Chem. Eng. J.271, 276–280 (2015). [Google Scholar]
- 31.Hu, Y. et al. Amino-functionalized multiple‐walled carbon nanotubes‐polyimide nanocomposite films fabricated by in situ polymerization. J. Appl. Polym. Sci.110(2), 701–705 (2008). [Google Scholar]
- 32.Babaei, S., Nematollahi, M. H. & Abedini, R. Pure and mixed gas permeation study of silica incorporated polyurethane-urea membrane modified by Moca chain extender. Can. J. Chem. Eng.98, 1543–1557 (2020). [Google Scholar]
- 33.Zhu et al. Facile covalent crosslinking of zeolitic imidazolate framework/polydimethylsiloxane mixed matrix membrane for enhanced ethanol/water separation performance. ACS Sustain. Chem. Eng.8, 12664–12676 (2020). [Google Scholar]
- 34.Dai, Z. et al. Poly (1-trimethylsilyl-1-propyne)-based hybrid membranes: effects of various nanofillers and feed gas humidity on CO2 permeation. Membranes8(3), 76 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mozafari, M., Rahimpour, A. & Abedini, R. Exploiting the effects of zirconium-based metal organic framework decorated carbon nanofibers to improve CO2/CH4 separation performance of thin film nanocomposite membranes. J. Ind. Eng. Chem.85, 102–110 (2020). [Google Scholar]
- 36.Nematollahi, M. H., Dehaghani, A. H. S. & Abedini, R. CO2/CH4 separation with Poly (4-methyl-1-pentyne)(TPX) based mixed matrix membrane filled with Al2O3 nanoparticles. Korean J. Chem. Eng.33(2), 657–665 (2016). [Google Scholar]
- 37.Kalantari, S. et al. Superior interfacial design in ternary mixed matrix membranes to enhance the CO2 separation performance. Appl. Mater. Today. 18, 100491 (2020). [Google Scholar]
- 38.Pakizeh, M. et al. Preparation and characterization of dimethyldichlorosilane modified Si O2/PSf nanocomposite membrane. Korean J. Chem. Eng.30(3), 751–760 (2013). [Google Scholar]
- 39.Ghasemi Estahbanati, E., Omidkhah, M. & Ebadi Amooghin, A. Interfacial design of ternary mixed matrix membranes containing Pebax 1657/silver-nanopowder/[BMIM][BF4] for improved CO2 separation performance. ACS Appl. Mater. Interfaces. 9(11), 10094–10105 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Kheirtalab, M., Abedini, R. & Ghorbani, M. A novel ternary mixed matrix membrane comprising polyvinyl alcohol (PVA)-modified poly (ether-block-amide)(Pebax® 1657)/graphene oxide nanoparticles for CO2 separation. Process Saf. Environ. Prot.144, 208–224 (2020). [Google Scholar]
- 41.Song, Q. et al. Zeolitic imidazolate framework (ZIF-8) based polymer nanocomposite membranes for gas separation. Energy Environ. Sci.5(8), 8359–8369 (2012). [Google Scholar]
- 42.Nafisi, V. & Hägg, M. B. Development of dual layer of ZIF-8/PEBAX-2533 mixed matrix membrane for CO2 capture. J. Membr. Sci.459, 244–255 (2014). [Google Scholar]
- 43.Li, W., Samarasinghe, S. A. S. C. & Bae, T. H. Enhancing CO2/CH4 separation performance and mechanical strength of mixed-matrix membrane via combined use of graphene oxide and ZIF-8. J. Ind. Eng. Chem.67, 156–163 (2018). [Google Scholar]
- 44.Ban, Y. et al. Confinement of ionic liquids in nanocages: tailoring the molecular sieving properties of ZIF-8 for membrane‐based CO2 capture. Angew. Chem. Int. Ed.54(51), 15483–15487 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Jomekian, A. et al. Ionic liquid-modified Pebax® 1657 membrane filled by ZIF-8 particles for separation of CO2 from CH4, N2 and H2. J. Membr. Sci.524, 652–662 (2017). [Google Scholar]
- 46.Diestel, L. et al. Comparative permeation studies on three supported membranes: pure ZIF-8, pure polymethylphenylsiloxane, and mixed matrix membranes. Microporous Mesoporous Mater.189, 210–215 (2014). [Google Scholar]
- 47.Jusoh, N. et al. Transport properties of mixed matrix membranes encompassing zeolitic imidazolate framework 8 (ZIF-8) nanofiller and 6FDA-durene polymer: optimization of process variables for the separation of CO2 from CH4. J. Clean. Prod.149, 80–95 (2017). [Google Scholar]
- 48.Abedini, R., Omidkhah, M. & Dorosti, F. Highly permeable Poly (4-methyl-1-pentyne)/ NH2-MIL 53 (Al) mixed matrix membrane for CO2/CH4 separation. RSC Adv.4, 36522–36537 (2014). [Google Scholar]
- 49.Chen, K. et al. Enhanced CO2/CH4 separation performance of mixed-matrix membranes through dispersion of sorption-selective MOF nanocrystals. J. Membr. Sci.563, 360–370 (2018). [Google Scholar]
- 50.Murali, R. S. et al. Mixed matrix membranes of Pebax-1657 loaded with 4A zeolite for gaseous separations. Sep. Purif. Technol.129, 1–8 (2014). [Google Scholar]
- 51.Abedini, R., Omidkhah, M. & Dorosti, F. Hydrogen separation and purification with poly (4-methyl-1-pentyne)/MIL-53 mixed matrix membrane based on reverse selectivity. Int. J. Hydrog. Energy. 39(15), 7897–7909 (2014). [Google Scholar]
- 52.Zhao, D. et al. Poly (amide-6-b-ethylene oxide)/SAPO-34 mixed matrix membrane for CO2 separation. J. Energy Chem.23(2), 227–234 (2014). [Google Scholar]
- 53.Shao, L. & Chung, T. S. In situ fabrication of cross-linked PEO/silica reverse-selective membranes for hydrogen purification. Int. J. Hydrog. Energy. 34(15), 6492–6504 (2009). [Google Scholar]
- 54.Li, Y. & Chung, T. S. Molecular-level mixed matrix membranes comprising Pebax® and POSS for hydrogen purification via preferential CO2 removal. Int. J. Hydrog. Energy. 35(19), 10560–10568 (2010). [Google Scholar]
- 55.Chen, F. et al. Preparation of mixed matrix composite membrane for hydrogen purification by incorporating ZIF-8 nanoparticles modified with Tannic acid. Int. J. Hydrog. Energy. 45(12), 7444–7454 (2020). [Google Scholar]
- 56.Chua, M. L. et al. Polyetheramine–polyhedral oligomeric Silsesquioxane organic–inorganic hybrid membranes for CO2/H2 and CO2/N2 separation. J. Membr. Sci.385, 40–48 (2011). [Google Scholar]
- 57.Rezakazemi, M., Shahidi, K. & Mohammadi, T. Hydrogen separation and purification using crosslinkable PDMS/zeolite A nanoparticles mixed matrix membranes. Int. J. Hydrog. Energy. 37(19), 14576–14589 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.















