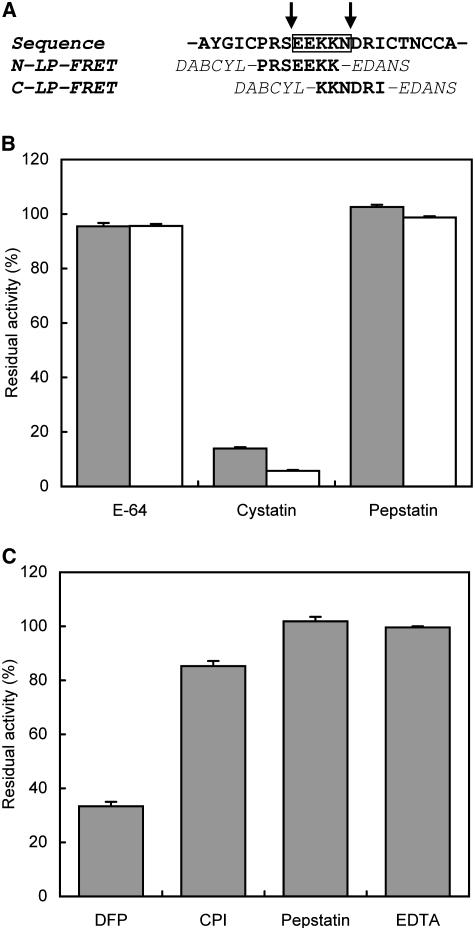
Figure 5.
Screening of proteases involved in processing of the LP. A, The substrates were designed based on the sequence of the LP (boxed) to separate two main processing sites (arrows) where in vivo proteolysis proceeds in the TPI precursor. The synthetic substrates were double labeled with DABCYL and EDANS for use in the FRET assay. B, The activity of VPE in leaf extract was screened with C-LP-FRET substrate (open bars) and specific substrate Z-AAN-AMC (closed bars). C, The activity of subtilisin-like protease in leaf extract was screened with the N-LP-FRET substrate. For testing the inhibitory sensitivity, we treated the extract with the class-selective protease inhibitors before the assay: E-64 (500 μm), cystatin (50 μm), pepstatin (10 μm), diisopropyl fluorophosphate (DFP; 10 mm), mixture of Cys protease inhibitors (CPI; 2.05 mm), and EDTA (1 mm). The inhibition is expressed as a percentage of residual activity relative to the uninhibited control.