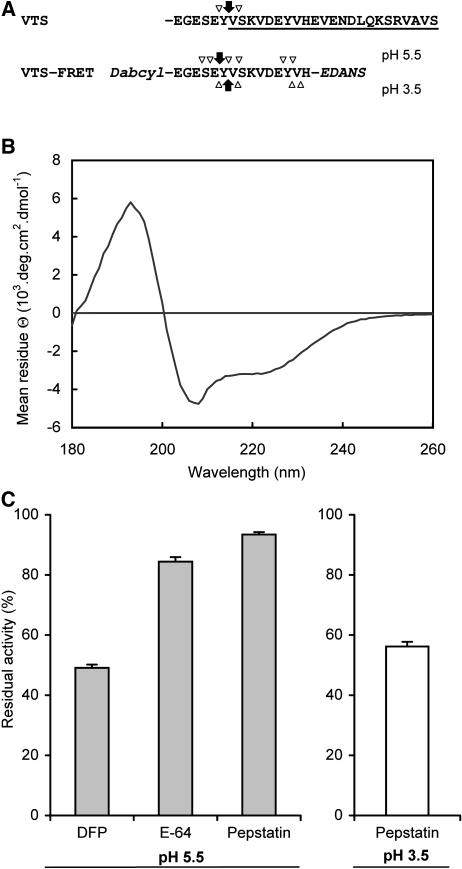
Figure 8.
Screening of proteases involved in the processing of VTS. A, The VTS-FRET substrate was designed based on the sequence of VTS (underline) surrounding the processing site where in vivo proteolysis proceeds in TPI precursor (arrows, major fragmentation; arrowheads minor fragmentation). The synthetic substrate was double labeled with DABCYL and EDANS for use in the FRET assay. The substrate was incubated with leaf extracts at pH 5.5 and 3.5, and the resulting fragments were identified by LC-MC. The major and minor bonds cleaved in VTS-FRET are labeled with arrows. B, Determination of helical conformation of VTS-FRET by circular dichroism spectroscopy. C, The proteolytic activity of leaf extracts (mean ± se) from three replicate plants was screened with VTS-FRET substrate at pH 5.5 and 3.5. The extracts were treated with the following class-selective protease inhibitors before the assay: diisopropyl fluorophosphate (DFP; 10 mm), E-64 (500 μm), and pepstatin (10 μm). The degree of inhibition was expressed in relation to the unelicited control leaves.