Abstract
Background
Primary liver cancer is one of the most common malignant tumors with a rising incidence in recent years. In China, it ranks as the fourth most prevalent malignancy. Although surgery is the primary treatment, many advanced-stage patients are ineligible due to late diagnosis, rapid progression, or other contraindications. Targeted therapy, chemotherapy, and transarterial chemoembolization (TACE) are common non-surgical approaches, but these often result in significant side effects such as gastrointestinal reactions, bone marrow suppression, and high recurrence rates. In this context, integrative treatment with traditional Chinese medicine (TCM) has shown promise. Cinobufacin, a TCM drug developed in China, has demonstrated superior efficacy and safety when combined with Western treatments in patients with advanced hepatocellular carcinoma (HCC).
Methods
We conducted a systematic review and meta-analysis by searching PubMed, Embase, Cochrane Library, CNKI, WanFang Data, VIP, and the Chinese Biomedical Literature Database (CBM) for randomized controlled trials (RCTs) comparing cinobufacin combined with conventional Western treatments to Western treatments alone in advanced HCC patients. The search covered all databases up to December 30, 2024. Statistical analyses were performed using Stata 15.0, and Review Manager.
Results
A total of 30 studies were included in the meta-analysis. Cinobufacin combination therapy significantly improved the disease control rate (DCR, OR = 2.49, 95% CI 2.01 to 3.07), reduced alpha-fetoprotein (AFP) levels (SMD = − 1.86, 95% CI − 2.58 to − 1.13), alanine aminotransferase (ALT) levels (SMD = − 1.66, 95% CI − 2.48 to − 0.83), and total bilirubin (TBIL) levels (SMD = − 1.82, 95% CI − 2.36 to − 1.28). It also increased white blood cell (WBC) counts (SMD = 0.58, 95% CI 0.06 to 1.11) and improved Karnofsky Performance Status (KPS) scores (SMD = 1.24, 95% CI 0.93 to 1.56). Funnel plots and sensitivity analyses confirmed the robustness of the results.
Conclusion
Cinobufacin combined with Western treatments significantly improves clinical efficacy, reduces adverse drug reactions, and enhances the quality of life for patients with advanced HCC.
Keywords: Advanced hepatocellular carcinoma, Cinobufacin, Efficacy, Meta-analysis, Safety
Introduction
Hepatocellular carcinoma (HCC) is one of the most common and lethal malignancies worldwide, accounting for a significant proportion of cancer-related deaths [1]. In China, HCC represents an even greater burden, ranking as the fourth most prevalent cancer and posing a major public health challenge [2]. Despite advancements in early detection and treatment, most patients are diagnosed at an advanced stage due to the asymptomatic nature of early disease and the rapid progression of HCC. This delayed diagnosis, combined with the tumor's aggressive nature, limits the eligibility of many patients for curative surgical interventions such as liver resection or transplantation [3]. For patients with advanced-stage HCC who are not candidates for surgery, non-surgical options, including targeted therapy, chemotherapy, and transarterial chemoembolization (TACE), remain the primary treatment modalities [4, 5]. While these therapies have demonstrated efficacy in controlling tumor progression and improving survival, they are often associated with significant limitations, including high recurrence rates, substantial gastrointestinal toxicity, bone marrow suppression, and other severe adverse effects [6, 7]. Consequently, therapeutic outcomes for advanced HCC remain suboptimal, highlighting the critical need for more effective and tolerable treatment strategies.
In recent years, there has been increasing interest in integrative approaches that combine traditional Chinese medicine (TCM) with Western medical treatments for HCC, particularly for patients with advanced-stage disease. These approaches aim to enhance therapeutic efficacy while minimizing the severe side effects of conventional therapies. Among the many TCM-based interventions, cinobufacin—a standardized Chinese medicinal preparation derived from the skin of Bufo bufo gargarizans—has garnered significant attention for its promising role in managing advanced HCC [8]. Cinobufacin has demonstrated the ability to improve treatment outcomes both as a standalone therapy and in combination with Western treatments such as TACE, chemotherapy (e.g., FOLFOX4), or supportive care [9]. Clinical studies suggest that cinobufacin can improve disease control rates, alleviate treatment-induced toxicity, and significantly enhance patients'quality of life [10, 11].
Despite the promising therapeutic potential of cinobufacin, there is a need for rigorous, evidence-based evaluation of its efficacy and safety when used in combination with Western medical treatments for advanced HCC. While individual studies have reported favorable outcomes, the lack of comprehensive synthesis and meta-analysis limits the ability to draw definitive conclusions about its clinical utility. A systematic evaluation of randomized controlled trials (RCTs) is essential to establish the role of cinobufacin in integrative treatment strategies and to provide robust evidence to guide clinical practice. The objective of this study is to perform a systematic review and meta-analysis of RCTs to assess the efficacy and safety of cinobufacin-based combination therapy in patients with advanced HCC.
Methods
Study design
This study is a systematic review and meta-analysis aimed at evaluating the efficacy and safety of cinobufacin-based combination therapy in the treatment of advanced HCC. By systematically synthesizing evidence from RCTs, the study seeks to provide a comprehensive assessment of the clinical outcomes associated with this integrative approach, focusing on both therapeutic effectiveness and the mitigation of adverse effects.
Eligibility criteria
Inclusion criteria
Study design: only RCTs were included to ensure the highest level of evidence. Population: studies involving patients diagnosed with advanced HCC. Intervention and control: the intervention group received cinobufacin-based combination therapy, while the control group received conventional Western treatments alone. Outcomes: studies reporting at least one of the following outcomes were included: disease control rate (DCR). Liver function markers, such as alanine aminotransferase (ALT) and total bilirubin (TBIL). Quality of life indicators, such as the Karnofsky Performance Status (KPS). Safety outcomes, including adverse effects and toxicity data.
Exclusion criteria
Study design: non-RCTs, such as observational studies, case reports, review articles, and editorials, were excluded. Data availability: studies with incomplete or unavailable data, such as missing key outcomes or insufficient statistical information, were excluded to maintain the reliability of the analysis.
Search strategy
A systematic search was conducted across multiple databases to identify relevant studies. The databases included PubMed, Embase, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), WanFang Data, VIP Chinese Science and Technology Journals Database, and Chinese Biomedical Literature Database (CBM). The search strategy employed a combination of Medical Subject Headings (MeSH) terms, keywords, and their synonyms. Key search terms included “cinobufacin,” “hepatocellular carcinoma” OR “liver cancer,” “combination therapy,” and “randomized controlled trials” OR “RCTs.” Boolean operators (“AND,” “OR”) were applied to combine terms effectively, and filters were used to refine the search results. The search spanned all studies published from the inception of each database up to December 30, 2024.
Study selection
Two independent reviewers conducted an initial screening of titles and abstracts to identify studies that potentially met the eligibility criteria. Full-text articles were retrieved for studies deemed eligible or unclear during the title and abstract screening phase. The same two reviewers independently assessed the full-text articles to determine their inclusion based on the predefined eligibility criteria. Any discrepancies between the two reviewers during the screening or selection process were resolved through discussion.
Data extraction
The following information was systematically extracted from each included study: Study Characteristics: author names, publication year, study design, sample size, intervention details, and control group details. Patient Demographics: baseline characteristics of participants, including age, and gender. Clinical Outcomes: key efficacy metrics, including DCR, AFP levels, ALT, TBIL, WBC counts, and KPS scores.
Risk of bias assessment
The risk of bias in the included studies was evaluated using the Cochrane Risk of Bias tool. The assessment covered the following domains: Random Sequence Generation: evaluating whether the allocation sequence was generated appropriately to ensure randomization. Allocation Concealment: assessing whether the allocation process was concealed to prevent selection bias. Blinding: examining the blinding of participants, personnel, and outcome assessors to reduce performance and detection bias. Incomplete Outcome Data: evaluating the handling of missing data and the potential for attrition bias. Selective Reporting: checking for the presence of selective outcome reporting to identify potential reporting bias. Other Biases: assessing any other sources of bias that could affect the validity of the study results.
Statistical analysis
Statistical analyses were performed using Stata 15.0, and Review Manager software. The following methods were employed: odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for dichotomous outcomes, such as the DCR. Standardized mean differences (SMDs) with 95% CIs were used for continuous outcomes, including AFP levels, ALT, TBIL, WBC counts, and KPS scores. Cochran’s Q test and the I2 statistic were used to assess statistical heterogeneity among the included studies. Subgroup analyses were conducted to explore potential sources of heterogeneity, such as differences in treatment regimens or patient characteristics. Sensitivity analysis was performed by sequentially excluding individual studies to test the robustness of the pooled results and identify potential outliers. Publication bias was assessed using funnel plots for visual inspection and Egger’s test for quantitative evaluation.
Ethical considerations
No ethical approval was required for this study, as it exclusively utilized data from previously published studies. No new patient data were collected, and no direct interventions were performed.
Reporting standards
The study was conducted and reported in strict adherence to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to ensure transparency, reproducibility, and methodological rigor.
Results
A total of 936 records were initially identified through database searches, as shown in Fig. 1. After the removal of 569 duplicates, 367 articles remained for further screening. Of these, 536 articles were excluded based on title and abstract review due to irrelevance or insufficient information. Full-text screening of the remaining 33 articles resulted in the exclusion of 3 studies (2 review articles and 1 article with unavailable full text), leaving 30 RCTs for inclusion in the meta-analysis.
Fig. 1.
PRISMA flow diagram
The characteristics of the included studies are summarized in Table 1. All studies were conducted in China and involved advanced HCC patients. The total sample size across the studies was substantial, with a range from 38 to 110 participants per study. The intervention groups received cinobufacin-based combination therapies, primarily combined with TACE (transarterial chemoembolization), and were compared to control groups receiving conventional Western therapies such as TACE alone, chemotherapy, or other standard treatments. The evaluation indices varied among studies and included DCR, liver function markers (e.g., ALT, TBIL), AFP levels, WBC counts, KPS scores, and adverse events. The demographic profiles of the patients were comparable between groups, with mean ages ranging from 42 to 65 years and a predominance of male participants in both intervention and control groups.
Table 1.
Characteristics of all studies
| No | Author | Year | Study design, location | Sample Size | Medication (group size) | Age (years) | Gender (male/%) | Evaluation index | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | ||||||
| 1 | Cheng Jianshan | 2024 | RCT, China | 108 | Huachansu tablets combined with TACE (n = 54) | TACE alone (n = 54) | 53.30 ± 10.33 | 52.80 ± 10.26 | 35 (64.81%) | 25 (46.30%) | OS; PFS |
| 2 | Li Jia | 2019 | RCT, China | 90 | Arterial catheterization of cinbufosin with TACE (n = 45) | TACE alone (n = 45) | 54 ± 8 | 50 ± 10 | 38 (84.4%) | 34 (75.5%) | DCR%; AFP; CA199; PFS; Adverse reaction |
| 3 | Jia Jinying | 2016 | RCT, China | 95 | Huachansu combined with TACE (n = 49) | TACE alone (n = 46) | 58.4 ± 8.3 | 58.1 ± 8.7 | 29 (59.1%) | 27 (58.6%) | DCR% |
| 4 | Deng Zhenyun | 2015 | RCT, China | 53 | Huachansu combined with TACE (n = 26) | TACE alone (n = 27) | 48.65 ± 16.12 | 48.30 ± 16.24 | 14 (56%) | 15 (62.5%) | DCR%; AFP |
| 5 | Wang Yifa | 2014 | RCT, China | 71 | Huachansu combined with TACE (n = 36) | TACE alone (n = 35) | 53 ± 8 | 52 ± 10 | 23 (63.8%) | 22 (62.8%) | DCR% |
| 6 | Liang Yong | 2008 | RCT, China | 96 | Huachansu combined with TACE (n = 48) | TACE alone (n = 48) | 51 ± 6 | 52 ± 8 | 35 (72.9%) | 37 (77%) | DCR% |
| 7 | Ke Jun | 2011 | RCT, China | 78 | Huachansu combined with TACE (n = 38) | TACE alone (n = 40) | 58.32 ± 11.59 | 57.09 ± 11.77 | 34 (89.4%) | 35 (87.5%) | DCR%; TBIL; ALT; AFP; WBC |
| 8 | Zhang Xiaoyu | 2011 | RCT, China | 60 | Huachansu combined with γ-knife (n = 30) | γ-Knife alone (n = 30) | 58.81 ± 14.45 | 57.29 ± 12.87 | 22 (73.3%) | 20 (66.7%) | DCR%; ALT; TBIL; AFP; KPS score; WBC |
| 9 | Wu Hongmei | 2000 | RCT, China | 80 | Huachansu combined with TACE (n = 36) | TACE alone (n = 44) | 52.4 ± 7.2 | 50.5 ± 8.7 | 31 (86.1%) | 38 (86.3%) | DCR%; AFP; ALT |
| 10 | Tian Siyuan | 2012 | RCT, China | 60 | Huachansu combined with FOLFOX4 (n = 30) | FOLFOX4 alone (n = 30) | 55.8 ± 5.4 | 56.3 ± 4.8 | 17 (56.6%) | 18 (60%) | DCR%; KPS score |
| 11 | Feng Xianming | 2012 | RCT, China | 70 | Huachansu combined with FOLFOX4 (n = 38) | FOLFOX4 alone (n = 32) | 52.6 ± 11.7 | 53.2 ± 10.4 | 21 (55.2%) | 23 (71.8%) | DCR%; WBC |
| 12 | Zhou Jiansheng | 2006 | RCT, China | 43 | Huachansu combined with TACE (n = 21) | TACE alone (n = 22) | 58.6 ± 8.5 | 58.5 ± 8.8 | 17 (80.9%) | 17 (77.2%) | DCR%; AST; ALT; ALB; TBIL; AFP; KPS score |
| 13 | Wei Youliang | 2013 | RCT, China | 110 | Huachansu combined with Seldinger (n = 55) | Seldinger alone (n = 55) | 46.81 ± 10.24 | 45.69 ± 9.84 | 37 (67.2%) | 40 (72.7%) | DCR% |
| 14 | Huang Zhifen | 2009 | RCT, China | 60 | Huachansu combined with TACE (n = 30) | TACE alone (n = 30) | 42.2 ± 5.3 | 41.1 ± 4.6 | 25 (83.3%) | 27 (90%) | DCR% |
| 15 | Sun Dexi | 2019 | RCT, China | 56 | Huachansu combined with TACE (n = 28) | TACE alone (n = 28) | 65.4 ± 5.4 | 63.6 ± 5.8 | 19 (57.1%) | 21 (75%) | DCR% |
| 16 | Yu Jianguo | 2013 | RCT, China | 60 | Huachansu combined with TACE (n = 30) | TACE alone (n = 30) | 49.7 ± 5.8 | 50.8 ± 4.7 | 14 (46.6%) | 15 (50%) | DCR%; AST; ALT; ALB; TBIL |
| 17 | He Shengli | 2012 | RCT, China | 51 | Huachansu combined with TACE (n = 26) | TACE alone (n = 25) | 58.5 ± 5.3 | 58.5 ± 5.5 | 20 (76.9%) | 18 (72%) | DCR%; AFP |
| 18 | Yan Min | 2010 | RCT, China | 60 | Huachansu combined with TACE (n = 30) | TACE alone (n = 30) | 65.4 ± 5.6 | 63.6 ± 5.8 | 17 (56.7%) | 23 (76.6%) | DCR% |
| 19 | Wang Li | 2020 | RCT, China | 84 | Huachansu combined with TACE (n = 42) | TACE alone (n = 42) | 59.6 ± 12.2 | 58.2 ± 13.1 | 29 (69%) | 26 (61.9%) | DCR%; TBIL; AFP |
| 20 | Xie Jin | 2014 | RCT, China | 60 | Huachansu combined with TACE (n = 30) | TACE alone (n = 30) | 47 ± 3 | 49 ± 4 | 18 (60%) | 14 (46.6%) | DCR% |
| 21 | Zhou Xiaobin | 2010 | RCT, China | 58 | Huachansu combined with usual care (n = 29) | Usual care (n = 29) | 48 ± 6 | 48 ± 8 | 20 (68.9%) | 17 (58.6%) | DCR%; ALT; TBIL; AFP; WBC |
| 22 | Feng Lihua | 2012 | RCT, China | 59 | Sorafenib combined with chinalbufin tablet treatment group (n = 30) | Sorafenib alone (n = 29) | 56.4 ± 5.3 | 55.8 ± 6.2 | 25 (83.3%) | 25 (86.2%) | DCR%; KPS score |
| 23 | Liu Yongqiang | 2010 | RCT, China | 82 | Huachansu combined with TACE (n = 38) | TACE alone (n = 44) | 54.21 ± 10.32 | 55.32 ± 11.62 | 34 (89.4%) | 38 (86.3%) | DCR%; OS; PFS |
| 24 | Li Wenhua | 2006 | RCT, China | 38 | Huachansu combined with TACE (n = 19) | TACE alone (n = 19) | 45 ± 5 | 45 ± 6 | 14 (73.6%) | 14 (73.6%) | DCR%; WBC; AFP; KPS score; OS; PFS |
| 25 | Liu Zhaomin | 2023 | RCT, China | 96 | Intraperitoneally injected bevacizumab and cisplatin combined with oral bufosin tablets (n = 48) | Intraperitoneally injected bevacizumab and cisplatin (n = 48) | 58.39 ± 4.15 | 57.68 ± 4.07 | 27 (56.25%) | 24 (50%) | DCR%; KPS score |
| 26 | Zhang Fulin | 2024 | RCT, China | 80 | Huachansu combined with TACE (n = 40) | TACE alone (n = 40) | 59.71 ± 5.10 | 57.13 ± 4.99 | 24 (60%) | 25 (62.5) | DCR%; VEGF-A; bFGF; Angplt |
| 27 | Yang Hai | 2024 | RCT, China | 100 | Huachansu combined with TACE (n = 50) | TACE alone (n = 50) | 58.46 ± 10.32 | 58.57 ± 10.48 | 31 (62%) | 32 (64%) | DCR%; AFP; ALT; AST; TBIL; KPS score |
| 28 | Zhang Wei | 2022 | RCT, China | 90 | Huachansu combined with TACE (n = 45) | TACE alone (n = 45) | 56.2 ± 10.7 | 55.8 ± 11.2 | 28 (62.2%) | 29 (64.4%) | DCR%; AFP; CA199;VEGF; OS; PFS |
| 29 | Wang Chen | 2022 | RCT, China | 106 | alpha interferon combined with cinbufosin capsule (n = 53) | Alpha interferon therapy (n = 53) | 51.81 ± 1.73 | 52.28 ± 1.89 | 37 (69.81%) | 38 (71.70%) | DCR%; AFP; OS; PFS |
| 30 | Tian Zhiping | 2024 | RCT, China | 100 | Huachansu combined with TACE (n = 50) | TACE alone (n = 50) | 55.1 ± 6.3 | 54.8 ± 6.3 | 41 (82%) | 40 (80%) | DCR%; VEGF; AFP; CA199 |
The quality of the 30 included RCTs was assessed using the Cochrane Risk of Bias (RoB) tool, as shown in Fig. 2. Overall, while the majority of studies were of sufficient methodological quality, some limitations, particularly in allocation concealment and blinding, indicate room for improvement in future trials.
Fig. 2.
Risk of bias assessment. A Summary of risk of bias for each included study based on the Cochrane Risk of Bias tool, categorized as low, unclear, or high risk. B Risk of bias graph showing the percentage of studies with low, unclear, and high risk of bias across various domains, including random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective reporting
Disease control rate
The DCR was reported in all 30 included studies, and the results are presented in Fig. 3. The pooled analysis demonstrated a significant improvement in DCR for the cinobufacin-based combination therapy group compared to the control group (OR = 2.49, 95% CI 2.01 to 3.07, p < 0.001). No substantial heterogeneity was observed across the studies (I2 = 0%, p = 0.985), indicating consistency in the reported effects. This suggests that cinobufacin, when combined with Western treatments, contributes to better disease control in patients with advanced hepatocellular carcinoma.
Fig. 3.
The forest plot shows the pooled OR for the DCR comparing cinobufacin-based combination therapy to control treatments in patients with advanced hepatocellular carcinoma
Alpha-fetoprotein
The effects of cinobufacin combination therapy on AFP levels are shown in Fig. 4. The meta-analysis revealed that the treatment significantly reduced AFP levels compared to the control group (SMD = − 1.86, 95% CI − 2.58 to − 1.13, p < 0.001). However, moderate to high heterogeneity was observed (I2 = 95.1%, p < 0.001), likely due to differences in baseline AFP levels and treatment protocols among the included studies.
Fig. 4.
The forest plot illustrates the SMD for AFP levels between the intervention and control groups
Liver function markers
Cinobufacin-based therapies demonstrated a significant reduction in liver function markers, including ALT and TBIL. For ALT levels (Fig. 5), the pooled SMD was − 1.66 (95% CI − 2.48 to − 0.83, p < 0.001), with high heterogeneity (I2 = 92.0%, p < 0.001). Similarly, for TBIL levels (Fig. 6), the pooled SMD was − 1.82 (95% CI − 2.36 to − 1.28, p < 0.001), with moderate heterogeneity (I2 = 80.5%, p < 0.001). These findings suggest that cinobufacin improves liver function by reducing liver enzyme levels and bilirubin, thereby mitigating liver damage in advanced HCC patients.
Fig. 5.
The forest plot depicts the SMD for ALT levels between the groups
Fig. 6.
The forest plot presents the pooled SMD for TBIL levels
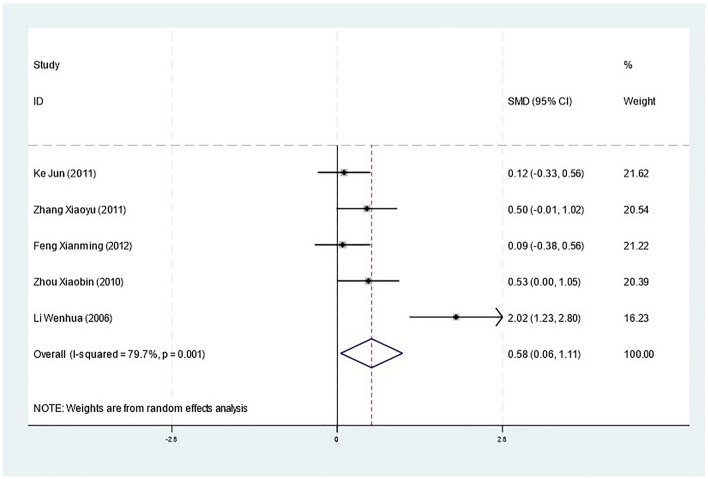
White blood cell count
Cinobufacin was also associated with an improvement in WBC counts, as shown in Fig. 7. The pooled analysis yielded a statistically significant SMD of 0.58 (95% CI 0.06 to 1.11, p = 0.027), with moderate heterogeneity (I2 = 79.7%, p = 0.001). This indicates that cinobufacin may enhance the immune function of patients, potentially contributing to its therapeutic effects.
Fig. 7.
The forest plot displays the SMD for WBC counts
Quality of life
The KPS score, a measure of quality of life, was significantly improved in the cinobufacin group compared to the control group, as shown in Fig. 8. The pooled SMD was 1.24 (95% CI 0.93 to 1.56, p < 0.001), with low to moderate heterogeneity (I2 = 57.3%, p = 0.029). This suggests that cinobufacin combination therapy not only improves clinical outcomes but also enhances the overall quality of life in patients with advanced HCC.
Fig. 8.
Forest plot of Karnofsky performance status (KPS) scores
Publication bias and sensitivity analysis
Publication bias was assessed using funnel plots and Egger’s test (Fig. 9). No significant asymmetry was observed, suggesting a low risk of publication bias in the included studies. Sensitivity analyses, conducted by sequentially excluding individual studies (Fig. 10), confirmed the robustness of the pooled results for all primary and secondary outcomes. The results remained consistent, indicating that the findings are reliable and not driven by any single study.
Fig. 9.
The funnel plot corresponds to the following outcomes: A DCR, B AFP levels, C ALT levels, D TBIL levels, E WBC counts, and F KPS scores
Fig. 10.
The sensitivity analysis corresponds to the following outcomes: A DCR, B AFP levels, C ALT levels, D TBIL levels, E WBC counts, and F KPS scores
Discussion
This meta-analysis demonstrated that cinobufacin-based combination therapy significantly improved clinical outcomes in patients with advanced HCC. The therapy was associated with a higher DCR compared to conventional treatments, along with notable reductions in key biomarkers, including AFP, ALT, and TBIL levels. Additionally, cinobufacin improved immune function, as evidenced by increased WBC counts, and enhanced patients’ quality of life, reflected in higher KPS scores [12]. The robustness of the findings is further supported by low heterogeneity in primary outcomes, particularly for DCR, and the consistency of results across sensitivity analyses, indicating that the conclusions are reliable and not influenced by any single study. These findings highlight the potential of cinobufacin-based combination therapy as an effective and safer integrative approach for the management of advanced HCC.
Comparison with previous studies
This meta-analysis confirms the efficacy of cinobufacin in improving clinical outcomes and reducing toxicity in advanced cancer treatments, consistent with previous research. Studies have shown that cinobufacin combined with TACE significantly increases objective response rates and 2-year survival rates in advanced hepatocellular carcinoma patients [9]. In advanced gastric cancer, cinobufacin injection enhances chemotherapy efficacy, improves quality of life, and alleviates side effects [10]. For advanced breast cancer, cinobufacin combined with chemotherapy improves clinical efficacy, reduces tumor markers, and decreases adverse reactions [13]. In moderate to advanced primary liver cancer, cinobufacin injection demonstrates beneficial effects on total response rates and 1–2 years survival rates [14]. These findings consistently support the use of cinobufacin as an adjunct therapy in various advanced cancers, improving treatment outcomes and reducing toxicity.
Mechanisms of action
The observed benefits of cinobufacin in advanced HCC can be attributed to several potential mechanisms of action:
Immune Modulation: studies have shown that cinobufacin stimulates the proliferation of splenocytes and macrophages, enhances phagocytic activity, and increases CD4+/CD8+ T-cell ratios [15]. It also promotes the secretion of Th1 cytokines while suppressing Th2 cytokines, potentially shifting the immune response towards tumor suppression [16]. Cinobufacin's anticancer properties include inducing tumor cell apoptosis, inhibiting proliferation and metastasis, and reversing multidrug resistance [17]. In clinical trials, combining cinobufacin with platinum-based chemotherapy for non-small-cell lung cancer improved disease control rates, survival rates, and immune function markers such as CD3+ and CD4+ T-cell counts, while reducing chemotherapy-related toxicity [18]. These findings suggest cinobufacin's promise as an immunotherapeutic agent for cancer treatment.
Hepatitis B Virus Suppression: chronic hepatitis B virus (HBV) infection is a major risk factor for hepatocellular carcinoma (HCC), particularly in Asia [19]. Antiviral therapies have shown promise in reducing HCC risk and improving outcomes. Studies have demonstrated that antiviral treatment can lower HCC incidence in chronic HBV patients, with one US-based study reporting a 61% risk reduction [20]. Antiviral therapies can inhibit HBV replication, reduce viral load, and improve liver function, potentially slowing tumor progression and enhancing treatment outcomes [21]. Cinobufacini, a traditional Chinese medicine, has exhibited anti-HBV properties in vitro, effectively inhibiting HBV antigen secretion and viral mRNA expression in HepG2.2.15 cells [22]. These findings suggest that antiviral treatments, including both conventional therapies and traditional medicines like cinobufacini, may play a crucial role in preventing HCC development and recurrence in chronic HBV patients.
Anti-Tumor Effects: cinobufacin exhibits direct cytotoxic effects on tumor cells by inducing apoptosis and inhibiting cell proliferation. It directly inhibits cancer cell proliferation and induces apoptosis through various mechanisms, including DNA damage, mitochondrial pathway activation, and cell cycle arrest [23]. Cinobufagin also disrupts angiogenesis by downregulating pro-angiogenic factors like VEGF and suppressing the endothelial mTOR/HIF-1α pathway, thereby impairing tumor blood supply and growth [24]. Its anti-angiogenic effects are mediated through ROS accumulation and mitochondrial dysfunction in endothelial cells [24]. Additionally, cinobufagin downregulates topoisomerase I and II expression, contributing to its antiproliferative effects [25].
These multifaceted mechanisms likely work synergistically to improve clinical outcomes and reduce treatment-related toxicity in HCC patients receiving cinobufacin-based combination therapy, making it a promising integrative treatment option.
Strengths of the study
This meta-analysis possesses several strengths that enhance the reliability and validity of its findings: a thorough and systematic search was conducted across multiple major databases, including both English and Chinese-language sources, ensuring the inclusion of a wide range of studies. The large number of RCTs incorporated into the analysis provides a robust evidence base for evaluating the efficacy and safety of cinobufacin-based combination therapy in advanced HCC. The study employed a well-defined and transparent methodological approach, including independent screening of studies, data extraction, and risk of bias assessment by two reviewers. Sophisticated statistical techniques were applied to synthesize the data, including ORs for dichotomous outcomes and SMDs for continuous outcomes. Heterogeneity was systematically assessed using Cochran’s Q test and I2 statistics, and subgroup and sensitivity analyses were performed to explore sources of variability and confirm the robustness of the findings.
Limitations
While this meta-analysis provides valuable insights, several limitations should be acknowledged:
Geographic Restriction: all included studies in this meta-analysis were conducted in China, where cinobufacin is widely utilized as part of traditional Chinese medicine. While the findings demonstrate promising efficacy for cinobufacin-based combination therapy in advanced HCC, the geographic restriction raises concerns regarding the generalizability of the results to other populations. Several factors may contribute to this limitation. First, healthcare systems and clinical practices in China may differ significantly from those in other regions, potentially influencing treatment protocols and patient outcomes. Second, patient demographics, including genetic predispositions, lifestyle habits, and the prevalence of underlying conditions such as chronic hepatitis B, vary across populations and could impact the therapeutic effects of cinobufacin. Although the results are encouraging, caution is warranted when extrapolating these findings to populations outside of China. Further studies in diverse geographic and clinical settings are needed to validate whether the benefits of cinobufacin-based therapy extend to other ethnic and demographic groups.
Variability in Treatment Protocols: there was notable variability in the included studies with respect to cinobufacin dosage, types of combination therapies (e.g., with TACE, chemotherapy, or supportive care), and control treatments. These differences may contribute to heterogeneity in certain outcomes, such as AFP and ALT levels, and could influence the consistency of the observed effects.
Potential Publication Bias: despite the use of funnel plots and Egger’s test, the possibility of publication bias cannot be entirely excluded. Studies with negative or insignificant results may have gone unpublished, potentially overestimating the overall efficacy and safety of cinobufacin-based combination therapy.
Lack of Long-Term Follow-Up Data: a notable limitation of the included studies is the relatively short follow-up periods, which restricts the assessment of the long-term efficacy and safety of cinobufacin-based combination therapy. While short-term outcomes, such as disease control rates, liver function, immune response, and quality of life, demonstrated consistent improvements, critical long-term outcomes—including overall survival (OS) and progression-free survival (PFS)—were reported in only a subset of studies. This gap in evidence raises concerns about the durability of the observed therapeutic benefits and whether they translate into meaningful survival advantages for patients with advanced HCC. Furthermore, the lack of long-term data limits the ability to evaluate potential late-onset adverse effects or risks associated with cinobufacin therapy. To address these limitations, future studies with extended follow-up durations are essential to comprehensively assess the sustained clinical benefits and long-term safety profile of cinobufacin-based regimens. Such research will provide a more robust evidence base to guide clinical decision-making in the treatment of advanced HCC. These limitations highlight the need for further research to validate the findings in more diverse populations, explore standardized treatment protocols, and assess long-term outcomes of cinobufacin combination therapy in advanced hepatocellular carcinoma.
Clinical implications
The findings of this meta-analysis have important practical implications for the treatment of advanced HCC. Cinobufacin-based combination therapy demonstrated significant improvements in therapeutic outcomes while reducing the toxicity associated with conventional treatments. These results support the integration of cinobufacin into current treatment protocols for advanced HCC as a complementary approach to enhance efficacy and improve patient quality of life.
Moreover, cinobufacin holds particular promise as a cost-effective treatment option in resource-limited settings, where access to advanced Western therapies such as targeted therapy or immunotherapy may be constrained by financial or logistical barriers. As a widely available and affordable traditional Chinese medicine, cinobufacin provides an alternative that can bridge the gap in cancer care, particularly in regions with high HCC burden and limited healthcare resources. These findings underscore the potential of integrative medicine to offer practical and scalable solutions for managing advanced HCC globally.
Conclusion
This meta-analysis demonstrates that cinobufacin-based combination therapy significantly improves clinical outcomes, including disease control rate, liver function, immune response, and quality of life in patients with advanced HCC. Despite some limitations, the findings support the integration of cinobufacin into treatment protocols as a complementary therapy, particularly in resource-limited settings. Further large-scale, multicenter studies are needed to validate these results, explore long-term benefits, and optimize treatment regimens.
Acknowledgements
Not applicable.
Author contributions
Conceptualization and Supervision, Y. Zhang; Investigation, Y. Zou; Visualization, T. Zhong; Resources, Y. Zou; Writing—Original draft, Y. Zhong; Reviewing, Editing, and Funding Acquisition, J. Zhang.
Funding
No funding.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li Z, Zhao M, Qi X, Tang Y, Cheng S. Mechanisms of portal vein tumour thrombus formation and development in patients with hepatocellular carcinoma. J Cell Mol Med. 2023;27(15):2103–11. 10.1111/jcmm.17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu IT, Yen CS, Wang WL, Tsai HW, Chu CY, Chang MY, et al. Predict early recurrence of resectable hepatocellular carcinoma using multi-dimensional artificial intelligence analysis of liver fibrosis. Cancers (Basel). 2021. 10.3390/cancers13215323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li YK, Wu S, Wu YS, Zhang WH, Wang Y, Li YH, et al. Portal venous and hepatic arterial coefficients predict post-hepatectomy overall and recurrence-free survival in patients with hepatocellular carcinoma: a retrospective study. J Hepatocell Carcinoma. 2024;11:1389–402. 10.2147/JHC.S462168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park HJ, Choi G, Ha S, Kim Y, Choi MJ, Kim M, et al. MBP-11901 inhibits tumor growth of hepatocellular carcinoma through multitargeted inhibition of receptor tyrosine kinases. Cancers (Basel). 2022. 10.3390/cancers14081994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu YC, Yang YC, Ma D, Wang JQ, Hao FJ, Chen XX, et al. FOLFOX-HAIC combined with targeted immunotherapy for initially unresectable hepatocellular carcinoma: a real-world study. Front Immunol. 2024;15:1471017. 10.3389/fimmu.2024.1471017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanno H, Goto Y, Sasaki S, Fukutomi S, Hisaka T, Fujita F, et al. Geriatric nutritional risk index predicts prognosis in hepatocellular carcinoma after hepatectomy: a propensity score matching analysis. Sci Rep. 2021;11(1):9038. 10.1038/s41598-021-88254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Wang QB, Liang YB, Chen XM, Luo WL, Li YK, et al. Tumor-associated lymphatic vessel density is a reliable biomarker for prognosis of esophageal cancer after radical resection: a systemic review and meta-analysis. Front Immunol. 2024;15:1453482. 10.3389/fimmu.2024.1453482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han M, Yang G, Lin Q, Yang Y, Zhang H, Su Y. Determination of endogenous bufalin in serum of patients with hepatocellular carcinoma based on HPLC–MS/MS. Front Oncol. 2019;9:1572. 10.3389/fonc.2019.01572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu T, Sun R, Wang Z, Yang W, Shen S, Zhao Z. A meta-analysis of Cinobufacini combined with transcatheter arterial chemoembolization in the treatment of advanced hepatocellular carcinoma. J Cancer Res Ther. 2014;10(Suppl 1):60–4. 10.4103/0973-1482.139763. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Yuan Y, Xi Y, Xu X, Guo Q, Zheng H, et al. Cinobufacini injection improves the efficacy of chemotherapy on advanced stage gastric cancer: a systemic review and meta-analysis. Evid Based Complement Altern Med. 2018;2018:7362340. 10.1155/2018/7362340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun D, Xiaofeng Z, Zhe C, Qun L, Hua Y, Wei C, et al. Treatment of huge hepatocellular carcinoma using cinobufacini injection in transarterial chemoembolization: a retrospective study. Evid-Based Complement Altern Med. 2016;2016:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YK, Wang QB, Liang YB, Ke Y. CT measurement of blood perfusion in hepatocellular carcinoma: from basic principle, measurement methods to clinical application. Zhonghua Zhong Liu Za Zhi. 2024;46(10):940–7. 10.3760/cma.j.cn112152-20240605-00240. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Li D, Du K, Wang J. Efficacy and safety of cinobufacin combined with chemotherapy for advanced breast cancer: a systematic review and meta-analysis. Evid Based Complement Altern Med. 2020;2020:4953539. 10.1155/2020/4953539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong Z, et al. Cinobufacini injection for moderate and advanced primary liver cancer: A systematic review and meta-analysis. J Chin Pharm Sci. 2019;28(4):12. [Google Scholar]
- 15.Yu Y, Wang H, Meng X, Hao L, Fu Y, Fang L, et al. Immunomodulatory effects of cinobufagin on murine lymphocytes and macrophages. Evid Based Complement Altern Med. 2015;2015: 835263. 10.1155/2015/835263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang XL, Zhao GH, Zhang J, Shi QY, Guo WX, Tian XL, et al. Immunomodulatory effects of cinobufagin isolated from Chan Su on activation and cytokines secretion of immunocyte in vitro. J Asian Nat Prod Res. 2011;13(5):383–92. 10.1080/10286020.2011.565746. [DOI] [PubMed] [Google Scholar]
- 17.Dai CL, Zhang RJ, An P, Deng YQ, Rahman K, Zhang H. Cinobufagin: a promising therapeutic agent for cancer. J Pharm Pharmacol. 2023;75(9):1141–53. 10.1093/jpp/rgad059. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Zhu D, Wu Z, Bai L, Wang D, Xu Y, et al. Effect of Cinobufacini plus platinum-based chemotherapy regimen on the immune function of patients with non-small-cell lung cancer: a meta-analysis. Heliyon. 2023;9(10): e20349. 10.1016/j.heliyon.2023.e20349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Gao JY, Wang J, Cheng J. The impact of anti-HBV treatment on the occurrence and recurrence of hepatocellular carcinoma: focus on Asian studies. Discov Med. 2015;19(103):89–99. [PubMed] [Google Scholar]
- 20.Maqsood Q, Sumrin A, Iqbal M, Younas S, Hussain N, Mahnoor M, et al. Hepatitis C virus/Hepatitis B virus coinfection: current prospectives. Antivir Ther. 2023;28(4):211915403. 10.1177/13596535231189643. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YQ, Guo JS. Antiviral therapies for hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol. 2015;21(13):3860–6. 10.3748/wjg.v21.i13.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui X, Inagaki Y, Xu H, Wang D, Qi F, Kokudo N, et al. Anti-hepatitis B virus activities of cinobufacini and its active components bufalin and cinobufagin in HepG2.2.15 cells. Biol Pharm Bull. 2010;33(10):1728–32. 10.1248/bpb.33.1728. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Li Y, Li S, Wang T, Wang M, Wu H, et al. Cinobufacini injection delays hepatocellular carcinoma progression by regulating lipid metabolism via SREBP1 signaling pathway and affecting macrophage polarization. J Ethnopharmacol. 2024;321: 117472. 10.1016/j.jep.2023.117472. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Chen C, Dai Y, Huang C, Han Q, Jing L, et al. Cinobufagin suppresses colorectal cancer angiogenesis by disrupting the endothelial mammalian target of rapamycin/hypoxia-inducible factor 1α axis. Cancer Sci. 2019;110(5):1724–34. 10.1111/cas.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Ban LY, Su X, Gao S, Liu JW, Cui XN. Effects of cinobufacini injection on cell proliferation and the expression of topoisomerases in human HepG-2 hepatocellular carcinoma cells. Mol Med Rep. 2015;12(1):1598–604. 10.3892/mmr.2015.3552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.