Abstract
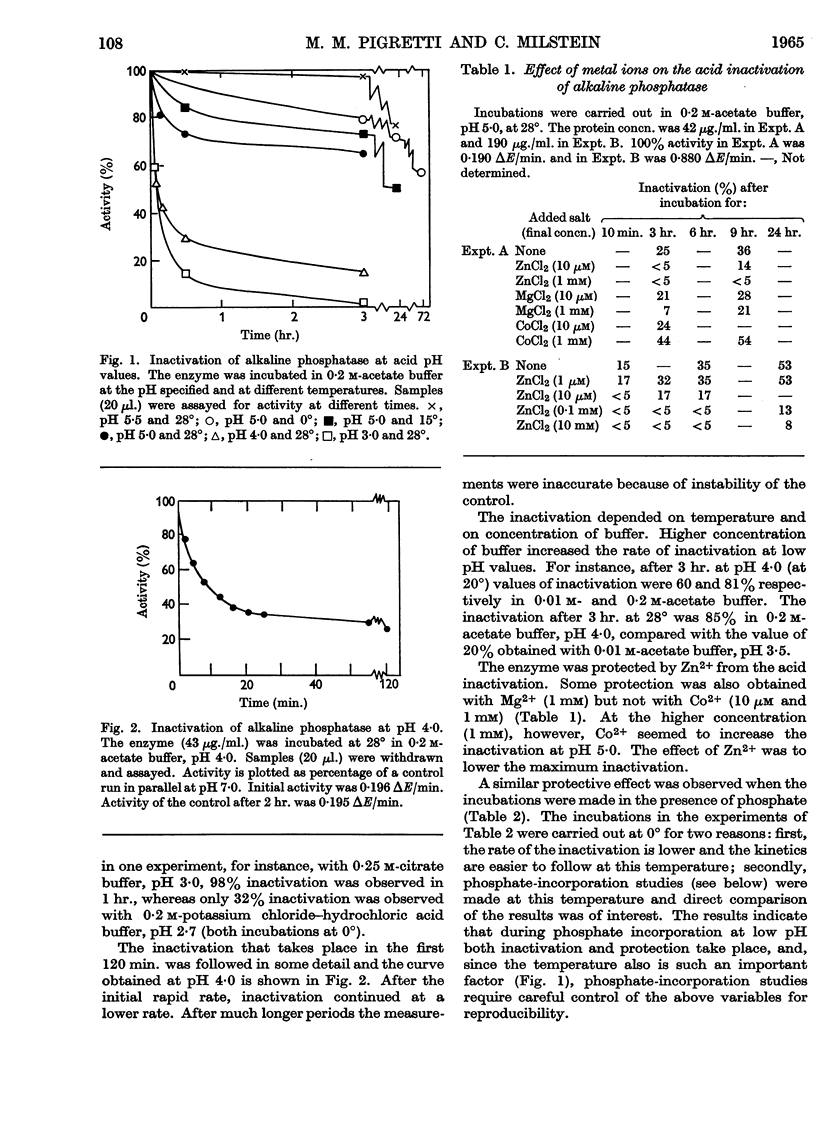
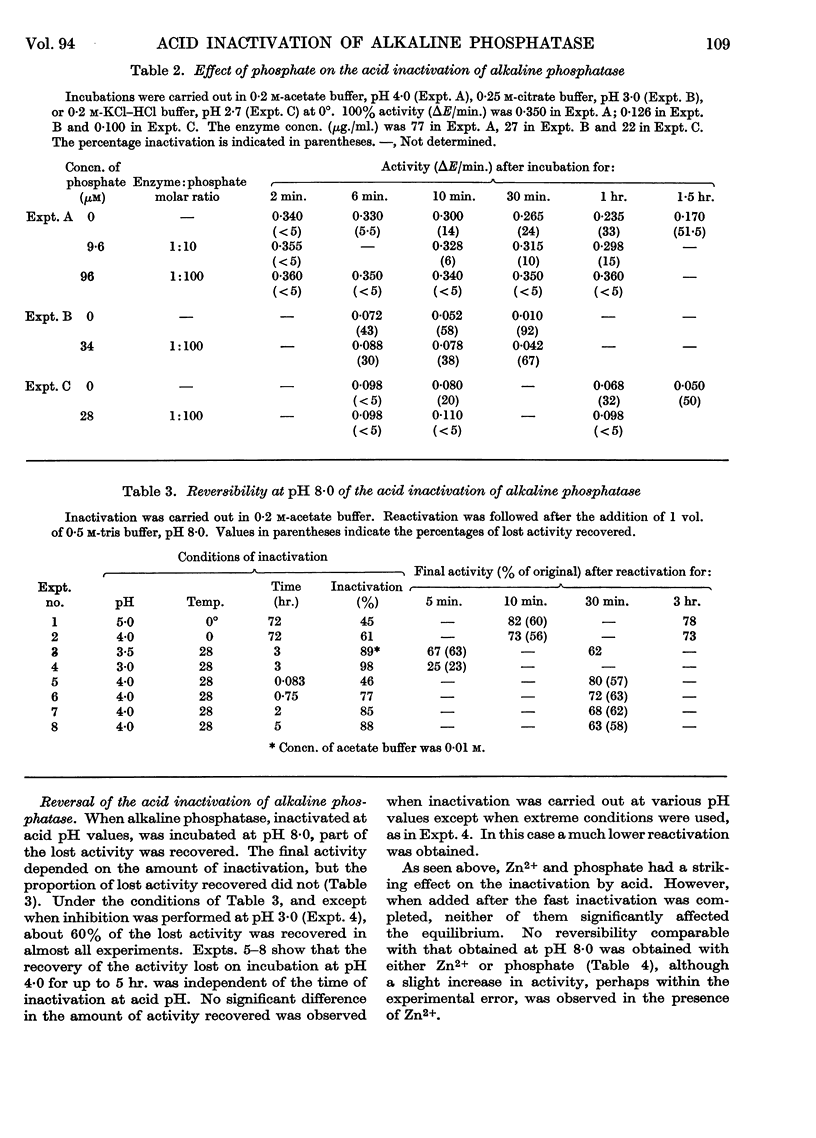
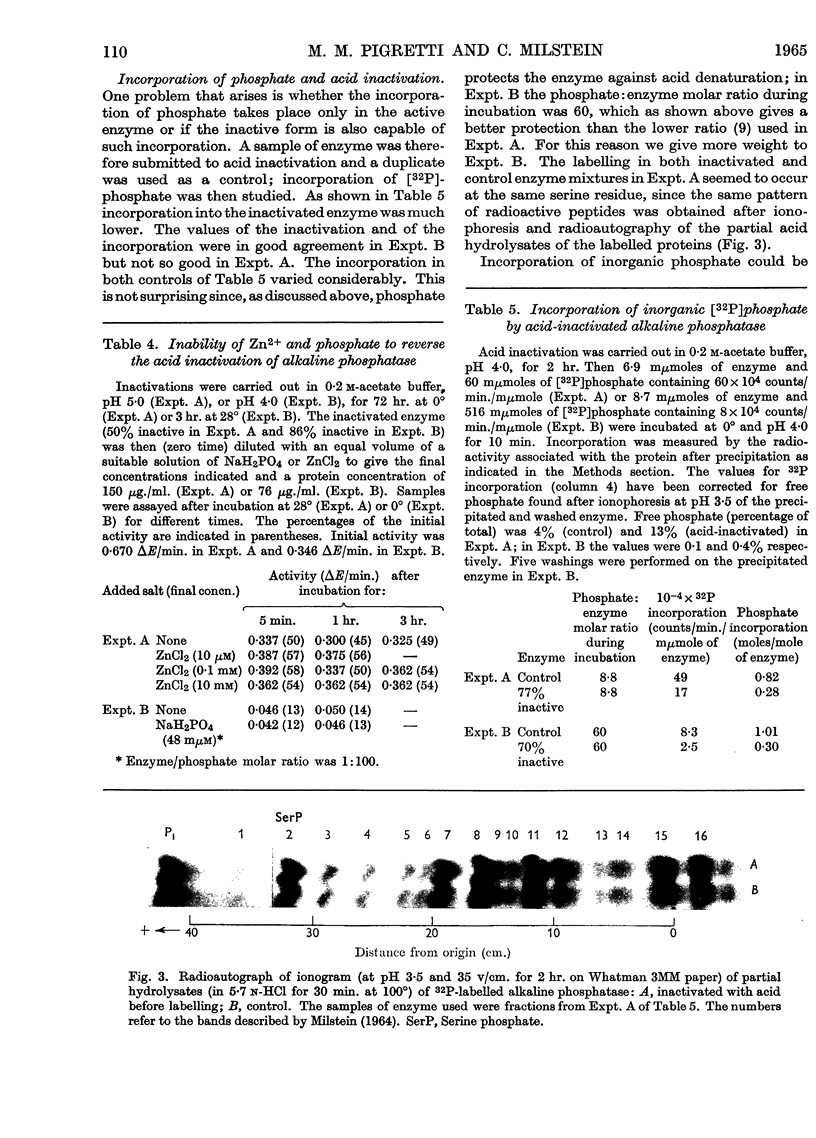
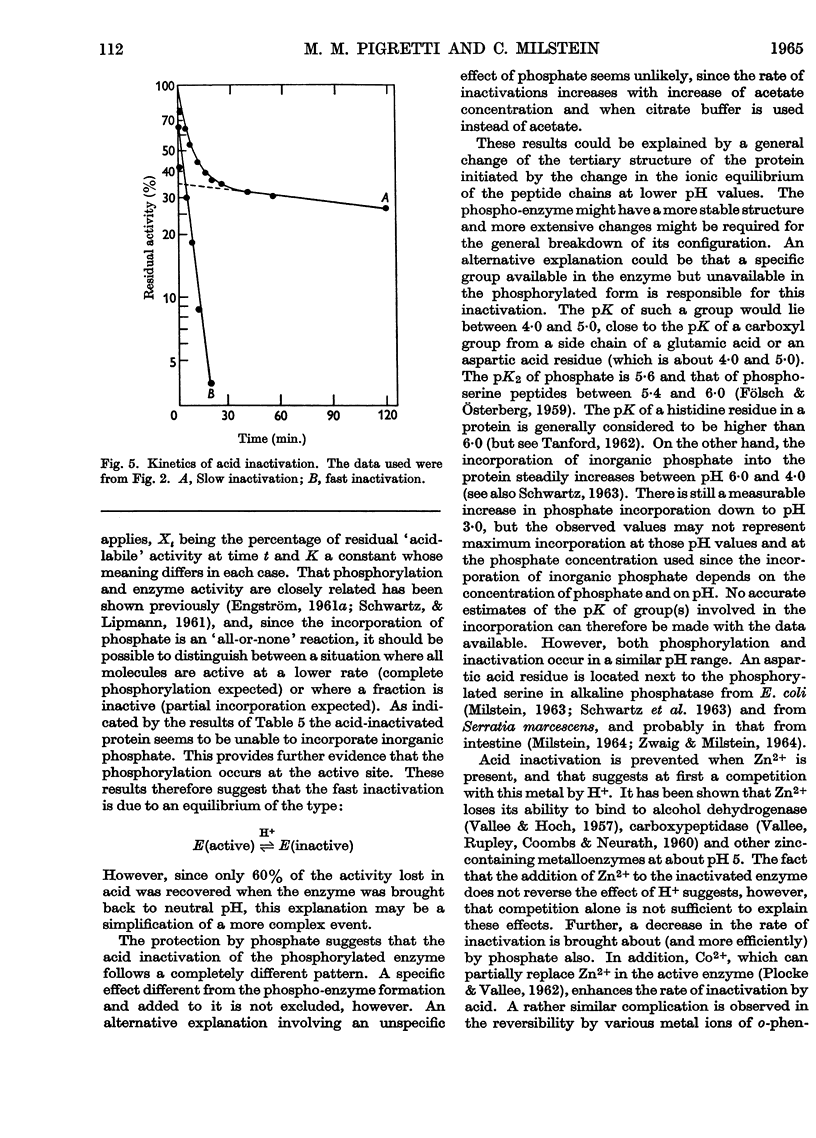
1. Alkaline phosphatase of Escherichia coli undergoes below pH 6·0 a reversible acid inactivation that has been studied and related to the extent of uptake of inorganic phosphate occurring below pH 6·0. 2. The rate of inactivation is rapid in the first few minutes but later it decreases markedly. Temperature, pH, composition of buffer and other factors have an important effect on the inactivation. 3. About 60% of the activity lost at pH values above 3·5 is rapidly recovered when the enzyme is taken back to pH 8·0, independently (within certain limits) of the extent of the inactivation. 4. Phosphate and Zn2+, although very good protectors of the inactivation by acid, are not by themselves able to reverse the acid inactivation. 5. Inorganic phosphate seems not to be incorporated into the acid-inactivated enzyme. 6. Incorporation of more than one mole of phosphate/mole of enzyme has been obtained, but the phosphate residues seem to be incorporated to serine residues with a common sequence, suggesting two identical active serine residues/molecule of active enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FOLSCH G., OSTERBERG R. The apparent acid ionization constants of some O-phosphorylated peptides and related compounds. J Biol Chem. 1959 Sep;234:2298–2303. [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- MILSTEIN C., SANGER F. An amino acid sequence in the active centre of phosphoglucomutase. Biochem J. 1961 Jun;79:456–469. doi: 10.1042/bj0790456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. The amino acid sequence around the reactive serine residue in alkaline phosphatase from Escherichia coli. Biochem J. 1964 Aug;92(2):410–421. doi: 10.1042/bj0920410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLOCKE D. J., LEVINTHAL C., VALLEE B. L. Alkaline phosphatase of Escherichia coli: a zinc metalloenzyme. Biochemistry. 1962 May 25;1:373–378. doi: 10.1021/bi00909a001. [DOI] [PubMed] [Google Scholar]
- PLOCKE D. J., VALLEE B. L. Interaction of alkaline phosphatase of E. coli with metal ions and chelating agents. Biochemistry. 1962 Nov;1:1039–1043. doi: 10.1021/bi00912a014. [DOI] [PubMed] [Google Scholar]
- RAY W. J., Jr, KOSHLAND D. E., Jr Identification of amino acids involved in phosphoglucomutase action. J Biol Chem. 1962 Aug;237:2493–2505. [PubMed] [Google Scholar]
- ROTHMAN F., BYRNE R. Fingerprint analysis of alkaline phosphatase of Escherichia coli K12. J Mol Biol. 1963 Apr;6:330–340. doi: 10.1016/s0022-2836(63)80092-3. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ J. H., LIPMANN F. Phosphate incorporation into alkaline phosphatase of E. coli. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1996–2005. doi: 10.1073/pnas.47.12.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H., Crestfield A. M., Lipmann F. THE AMINO ACID SEQUENCE OF A TETRADECAPEPTIDE CONTAINING THE REACTIVE SERINE IN E. COLI ALKALINE PHOSPHATASE. Proc Natl Acad Sci U S A. 1963 May;49(5):722–729. doi: 10.1073/pnas.49.5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALLEE B. L., HOCH F. L. Zinc in horse liver alcohol dehvdrogenase. J Biol Chem. 1957 Mar;225(1):185–195. [PubMed] [Google Scholar]
- Zwaig N., Milstein C. The amino acid sequence around the reactive serine residue in alkaline phosphatase of Serratia marcescens. Biochem J. 1964 Aug;92(2):421–422. doi: 10.1042/bj0920421. [DOI] [PMC free article] [PubMed] [Google Scholar]