Abstract
This study evaluated the clinical utility of 68Ga-DOTATATE PET/CT compared to 18F-FDG PET/CT for tumor staging and the delineation of primary tumor volume in patients with non-keratinizing nasopharyngeal carcinoma (NPC). Forty-two individuals with pathologically confirmed non-keratinizing NPC were recruited. This study compared the detection rates of primary and metastatic tumors and the accuracy of tumor staging using two PET/CT modalities. Tumor volumes defined on PET scans using the absolute SUV of 2.5 (TH2.5), 40% of the maximum SUV (TH40%), and the relative background-dependent threshold (THbgd) were analyzed in comparison to MRI results. Comparing 68Ga-DOTATATE and 18F-FDG PET/CT, identifying primary tumors (initial detection, 100% vs. 97.3%; recurrent detection, 80.0% vs. 100%) and lymph node metastases (99.0% vs. 100%) were comparable. However, 68Ga-DOTATATE PET/CT detected more skull base bone (100% vs. 96.3%) and intracranial invasion (100% vs. 54.5%) than 18F-FDG, and consequently correctly upwardly adjusted the T-staging in 7 patients. 68Ga-DOTATATE PET/CT detected an equal number of lung metastases (24/24) but more bone metastases (97.8% vs. 84.4%) compared to 18F-FDG PET/CT, yet was less effective for liver metastases (30.4% vs. 100%). Compared with 18F-FDG PET/CT, 68Ga-DOTATATE PET/CT correctly upstaged 5 subjects and downstaged 1 subject in overall staging. Tumor volumes assessed by 68Ga-DOTATATE PET compared to 18F-FDG PET using the three threshold methods demonstrated less variability and higher agreement with MRI. Among the methods, THbgd for lesion segmentation in 68Ga-DOTATATE PET demonstrated the highest confidence level and concordance with MRI (ICC 0.95). In conclusion, 68Ga-DOTATATE PET/CT is a beneficial complement to 18F-FDG PET/CT for NPC staging, with higher accuracy for primary tumor volume delineation.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-00625-y.
Keywords: 18F-FDG, 68Ga-DOTATATE, Nasopharyngeal cancer, PET/CT
Subject terms: Cancer imaging, Head and neck cancer, Oncology
Nasopharyngeal carcinoma (NPC) is the most prevalent epithelial malignancy in the area of the head and neck1. While NPC is relatively uncommon, it displays significant prevalence in particular regions, particularly East and Southeast Asia2. The insidious onset of NPC and its propensity for early metastasis result in many patients receiving diagnoses at advanced stages of the disease. The clinical stage of NPC significantly influences prognosis. Upon reaching an advanced stage, the patient’s mortality rate is increased considerably3. Therefore, early identification and accurate staging are essential to diagnosing and treating patients with NPC.
Integrating metabolic and anatomical data in 18F-FDG PET/CT offers substantial advantages over anatomical imaging alone. In patients with NPC, it enhances the accuracy of staging and the evaluation of therapeutic response. The presence of inflammatory and reactive hyperplastic lymph nodes in the neck, combined with the inflammatory response induced by radiotherapy and chemotherapy, complicates the accurate detection of local residual or recurrent tumors and lymph node metastasis using 18F-FDG PET/CT4. Radiation therapy is the primary therapeutic option for NPC. Target tumor volume depicted via 18F-FDG PET/CT correlates with radiotherapy efficacy5,6. Moreover, the tumor volume delineated based on 18F-FDG PET is highly dependent on the method used for PET signal segmentation, with different thresholds yielding different tumor volumes7–10. However, the lack of authoritative standardized uptake value (SUV) lesion segmentation methodologies considerably reduces the validity of tumor volumes determined by 18F-FDG PET/CT.
In areas with high NPC prevalence, non-keratinizing squamous carcinoma with Epstein-Barr virus (EBV) infection has the highest frequency (> 95%)1,11. EBV infection significantly raises NPC tissue somatostatin receptor 2 (SSTR2) expression12,13. [68Ga-DOTA, Tyr3]-octreotate (68Ga-DOTATATE) PET/CT targeting SSTR2 detected primary and metastatic NPC tumors effectively previously reported studies14,15. An optimal tumor background ratio enhances the visualization of the primary tumor. Thresholding methods represent commonly used techniques for quantifying biological target volumes in PET imaging. To our knowledge, the use of 68Ga-DOTATATE PET for assessing and defining primary tumor volumes in NPC has not been previously documented.
In this context, this study aimed to investigate further the efficacy of 68Ga-DOTATATE PET/CT in identifying primary and metastatic NPC and to evaluate its influence on tumor staging compared to 18F-FDG PET/CT. Moreover, focused segmentation was conducted employing various SUV threshold methodologies to assess the precision of primary tumor volume delineation using 68Ga-DOTATATE PET.
Methods
Participant cohort and study design
This single-center prospective study investigated 68Ga-DOTATATE PET/CT in non-keratinizing nasopharyngeal carcinoma. The Clinical Research Ethics Committee of the Affiliated Hospital of Southwest Medical University approved the study (Approval No. 2020035), adhering to the 1964 Declaration of Helsinki and its subsequent revisions. Every participant provided informed consent. Eligible patients were recruited at our facility from June 2022 to December 2022. In a week, all MR scanning, 68Ga-DOTATATE PET/CT, and 18F-FDG PET/CT were completed in this order, with no less than one day elapsed between the acquisitions of 68Ga-DOTATATE PET/CT and 18F-FDG PET/CT. The following criteria were applied to determine the study participants: (1) Patients with histopathologically proven non-keratinizing NPC who are either newly diagnosed or have previously had treatment; (2) Patients who have not received anti-tumor medication in the preceding three months. The exclusion criteria of the study were: (1) Patients with multiple primary malignant tumors; (2) Individuals for whom the examination was inappropriate.
18F-FDG and 68Ga-DOTATATE Preparation
Standard procedures were followed to synthesize 18F-FDG using an 18F-FDG-compliant synthesis module (FDG-N, PET Science & Technology). 68Ga-DOTATATE was synthesized using established methodologies16. Radiochemical purities of both were greater than 95%.
Imaging acquisition
The individuals fasted for at least 6 h before the 18F-FDG PET/CT to maintain normal blood glucose levels (3.9–6.1 mmol/L). The 68Ga-DOTATATE PET/CT requires no specific preparation. 68Ga-DOTATATE and 18F-FDG were administered intravenously at concentrations of 1.85 MBq/kg and 3.7 MBq/kg, respectively. The PET/CT imaging was conducted sixty minutes post-radiotracer administration. Every scan followed a previously published technique16,17, and a post-processing workstation (version R002, UWS-MI, United Imaging Medical) uploaded the collected data. The nasopharyngeal and neck contrast-enhanced MR exams were conducted using previously published techniques17.
Imaging analysis
Two nuclear medicine physicians (L.Q and Z.H) and two radiologists (P.L and G.C) independently and randomly interpreted PET/CT and MRI images. Reviewers were unaware of additional imaging data. For primary tumors, visual assessment of boundaries was performed by comparing radiotracer uptake at lesion margins with adjacent healthy tissue on PET images. The boundaries and extent of lesion invasion were identified when radiotracer uptake at the margin was significantly higher than background values. Lesion morphology identification and localization were aided by co-registered CT. Each primary tumor’s margins and extent were assessed, and any differences between MRI, 68Ga-DOTATATE PET/CT, and 18F-FDG PET/CT were documented. For metastatic tumors, lesions demonstrating radiotracer uptake above normal tissue background (with or without morphological abnormalities) were defined as positive after excluding physiological uptake and benign lesions on PET/CT. As per the 9th edition of the American Joint Committee on Cancer staging system, the clinical staging was determined based on 68Ga-DOTATATE PET/CT and 18F-FDG imaging methods18, respectively.
The diagnosis was confirmed by the lesion’s histopathology. For evaluating initial tumor infiltration, contrast-enhanced MRI was the gold standard19. Follow-up was acquired for metastatic lesions with unobtainable pathohistologic results. According to the RECIST 1.1 recommendations20, during follow-up, a lesion was considered malignant if multiple imaging examinations demonstrated classic signs of malignancy, and there was a significant increase (≥ 20% in longest diameter) or decrease (≥ 30% in longest diameter) in target lesion size following anti-tumor therapy.
Tumor volume delineation
The parameters were determined automatically after Hermes software identified tumor areas of interest on PET/CT and MR images. To determine the primary tumor volume on MRI, an area of interest was manually identified layer by layer along the lesion edge using enhanced T1WI images. The threshold techniques used to assess 18F-FDG and 68Ga-DOTATATE primary tumor volumes were21: (1) The SUV absolute threshold of 2.5 (TH2.5); (2) The SUV relative threshold of 40% of SUVmax (TH40%); (3) The relative background dependent threshold (THbgd). THbgd was calculated as SUV = SUVbgd + 20% (SUVmax - SUVbgd), where SUVbgd is the mean SUVmax of 10 randomly defined regions of interest in the non-pathological area surrounding the primary tumor, excluding the brain, salivary glands, oral cavity, and orbital region. The contours delineating the volume of interest are established on the PET image using the specified thresholds, and the contours surrounding the target lesion inside the defined boundary are automatically produced to compute the tumor volume.
Statistical analyses
The statistical analysis was performed with SPSS 25.0 (IBM). The quantitative data were presented as the mean ± standard deviation (SD) or median. The sensitivity of the two radiotracers was compared using McNemar’s test. The SUVmax of primary tumors and metastases was compared using 18F-FDG and 68Ga-DOTATATE PET/CT by the paired t-test and Mann-Whitney test. The intragroup correlation coefficient (ICC) was utilized to compare the consistency of the tumor volume between enhanced MRI and PET/CT at different thresholds. Two-tailed p < 0.05 indicated statistical significance.
Results
Patient characteristics
In the final stage, 42 participants were enrolled in the study, comprising 5 patients scheduled to have testing for metastasis or recurrence and 37 newly diagnosed patients. Participant characteristics are displayed in Table 1.
Table 1.
Patient characteristics.
| Characteristics | Number |
|---|---|
| No. patients | 42 |
| Age (year) | |
| Mean ± standard deviation | 49 ± 11 |
| Gender | |
| Male | 33 |
| Female | 9 |
| Histology, WHO type | |
| Diferentiated non-keratinizing carcinomas | 19 |
| Undiferentiated non-keratinizing carcinomas | 23 |
| Patient status | |
| Initial assessment | 37 |
| Recurrence or metastasis detection | 5 |
Comparison of 68Ga-DOTATATE PET/CT versus 18F-FDG PET/CT to assess the primary and metastatic lesions
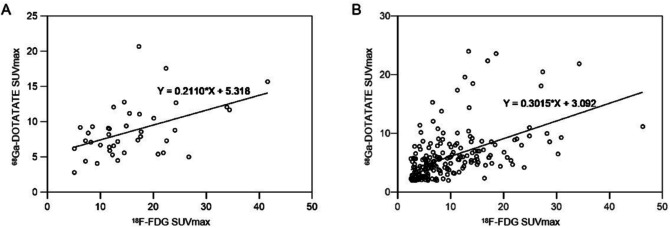
Table 2 compares lesion detection and radiotracer uptake between the two modalities. 68Ga-DOTATATE and 18F-FDG PET/CT had comparable primary tumor detection rates. In one newly diagnosed patient, 18F-FDG PET/CT and MRI were unable to identify the primary tumor (Fig. 1). One of the instances of primary tumor recurrence showed no positive radiotracer uptake on 68Ga-DOTATATE PET/CT 18F-FDG uptake was significantly higher in primary tumors than in 68Ga-DOTATATE. In 40 individuals, there was a moderate association between the two radiotracers’ absorption by the primary tumor (Fig. 2A). MRI revealed 27 cases of invasion of the skull base bone and 11 occurrences of intracranial invasion. 68Ga-DOTATATE PET/CT detected more skull base bone invasion (100% [27/27] vs. 96.3% [26/27]) and intracranial invasion (100% [11/11] vs. 54.5% [6/11]) than 18F-FDG. Compared to 18F-FDG, 68Ga-DOTATATE PET/CT revealed a larger lesion area in two invasions of the skull base bone and three intracranial invasions. The example in Fig. 3 demonstrated how the two approaches differed in determining primary tumor invasion.
Table 2.
Comparison of semi-quantitative parameters and lesions detection between 18F-FDG and 68Ga-DOTATATE PET/CT.
| Site of disease | No. of patients | No. of lesions | 18F-FDG uptake | 68Ga-DOTATATE uptake | P value (SUVmax) | P value (lesions detection) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| SUVmax | No. of Positive Lesions | Detection rates (%) | SUVmax | No. of Positive Lesions | Detection rates | |||||
| Primary tumors | 37 | 37 | 15.83 ± 8.31 | 36 | 97.3 | 8.79 ± 3.84 | 37 | 100% | < 0.001 | N |
| Local recurrence | 5 | 5 | 11.7* (7.7–26.7) | 5 | 100 | 7.4* (5.0-10.5) | 4 | 80% | N | N |
| Lymph nodes | 36 | 202 | 9.6 ± 7.05 | 202 | 100 | 6.0 ± 4.17 | 200 | 99.0% | < 0.001 | 0.05 |
| Distant metastases | ||||||||||
| Liver | 3 | 23 | 4.7* (3.4–25.5) | 23 | 100 | 10.0* (8.4–10.8) | 7 | 30.4% | 0.02 | <0.001 |
| Lung | 2 | 24 | 3.95* (1.4–25.9) | 24 | 100 | 1.65* (0.9–5.3) | 24 | 100% | < 0.001 | N |
| Bone | 5 | 45 | 11.1 ± 10.02 | 38 | 84.4 | 6.33 ± 3.74 | 44 | 97.8 | < 0.001 | 0.07 |
Data were presented as mean ± standard deviation. *median, N = not application.
Fig. 1.
68Ga-DOTATATE (A) and 18F-FDG PET/CT (B) images in a 37-year-old woman with NPC. 68Ga-DOTATATE PET/CT showed intensive tracer uptake in the primary tumor of the left pharyngeal recess (dotted arrow), while 18F-FDG PET/CT and MRI (C) showed no abnormal findings in the corresponding region (dotted arrows). Moreover, 68Ga-DOTATATE PET/CT revealed higher tracer uptake than 18F-FDG PET/CT in the right cervical (level IIB) lymph node (solid arrows).
Fig. 2.
Comparison of SUVmax of 68Ga-DOTATATE and 18F-FDG in matched double-positive primary tumors (A, r = 0.466, p = 0.002) and lymph nodes (B, r = 0.51, p = 0.000).
Fig. 3.
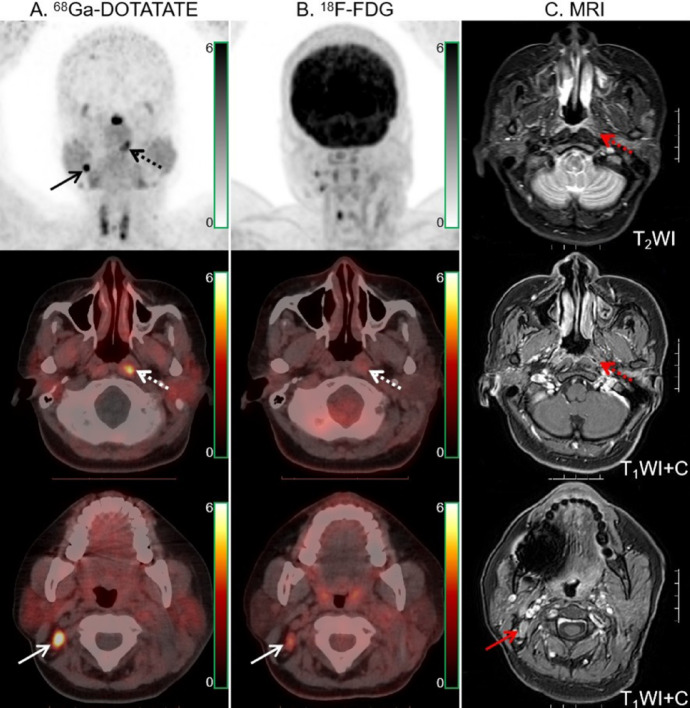
68Ga-DOTATATE (A) and 18F-FDG PET/CT (B) images in a 43-year-old man with NPC. Intense 68Ga-DOTATATE uptake was observed in the bilateral cavernous sinuses (dotted arrows), suggesting intracranial invasion, but 18F-FDG PET/CT revealed no abnormal intracranial 18F-FDG uptake (dotted arrows). Moreover, 68Ga-DOTATATE PET/CT showed strong tracer uptake in the sphenoid, occipital and petrous bones (solid arrow). The extent of skull base bone invasion (solid arrows) shown by 18F-FDG PET/CT was significantly smaller than that shown by 68Ga-DOTATATE PET/CT. MRI (C) findings were matched with 68Ga-DOTATATE PET/CT.
Thirty-six of the forty-two people were suspected of having lymph node metastases. The assessment comprised 221 lymph nodes in total. Histopathology was employed as the reference standard for 27 lymph nodes, while morphological analysis and/or imaging follow-up were used to confirm the remaining lymph nodes. Finally, 202 lymph nodes were found to have metastasized, while the remaining 19 were classified as reactive hyperplastic or inflammatory lymph nodes. 68Ga-DOTATATE PET/CT detection of lymph node metastases was comparable to 18F-FDG PET/CT 18F-FDG and 68Ga-DOTATATE PET/CT revealed 18 and 4 false-positive lymph nodes, respectively 18F-FDG uptake in lymph node metastases was greater than 68Ga-DOTATATE. The absorption of both radiotracers by lymph node metastases was moderately correlated (Fig. 2B).
Of the forty-two people, six had visceral or bone metastases. 68Ga-DOTATATE and 18F-FDG PET/CT detected all 24 lung metastases. Compared to 18F-FDG, 68Ga-DOTATATE PET/CT showed more bone metastases; however, the difference was not statistically significant (p = 0.07). Compared to 18F-FDG, 68Ga-DOTATATE PET/CT displayed a decreased detection rate of liver metastases. 68Ga-DOTATATE uptake was lower than 18F-FDG in the lung and bone metastases when evaluating radiotracer uptake by distant metastases. However, liver metastases had higher uptake of 68Ga-DOTATATE than 18F-FDG. A sample patient with distant metastases is presented in Fig. 4.
Fig. 4.
68Ga-DOTATATE (A) and 18F-FDG PET/CT (B) images in a 48-year-old woman with NPC. 68Ga-DOTATATE and 18F-FDG MIP images demonstrated multiple abnormal foci in the head, neck and trunk 18F-FDG PET/CT showed more hepatic metastases than those shown in 68Ga-DOTATATE PET/CT (solid arrows). The osteogenic metastasis (dotted arrows) revealed no significant 18F-FDG uptake but intense 68Ga-DOTATATE uptake.
Comparison of 68Ga-DOTATATE PET/CT versus 18F-FDG PET/CT for tumor staging
In seven cases, the T-stage was underestimated by 18F-FDG PET/CT owing to the physiologically enhanced radiotracer uptake by normal brain tissue. The T-staging of one patient was underestimated due to the inability of 68Ga-DOTATATE PET/CT to detect the return of the initial tumor. Furthermore, N-staging was overestimated by 18F-FDG and 68Ga-DOTATATE PET/CT in four and one patient, respectively. Moreover, in one case, the M-staging was underestimated by 18F-FDG PET/CT and was rectified by 68Ga-DOTATATE PET/CT.
Compared to 18F-FDG, while 68Ga-DOTATATE PET/CT resulted in inaccurate staging for one restaged patient, it correctly upstaged five patients (four from stage III to IVA and one from stage II to IVB) and correctly downstaged one patient (from stage III to II). Detailed clinical information and TNM staging for all patients are provided in the Supplementary Materials.
Comparison of primary tumor volume between 68Ga-DOTATATE and 18F-FDG PET/CT
Forty patients with primary tumors that could be detected were assessed. Tumor volumes and consistency analysis using various methods are displayed in Table 3. Tumor volume identified using various threshold techniques on 18F-FDG PET/CT differed considerably. Compared to 18F-FDG, 68Ga-DOTATATE PET demonstrated reduced differences in tumor volume, aligning more closely with enhanced MRI findings. Tumor volume measured using THbgd thresholding in 68Ga-DOTATATE PET was found to have the highest confidence and consistency with MRI (ICC: 0.95).
Table 3.
Intra-group correlation analysis of the primary tumor volume measured by contrast-enhanced MR and 18F-FDG PET/ 68Ga-DOTATATE PET with different thresholds.
| Modalities and thresholds | Tumor volume (cm3) | ICC | 95% CI |
|---|---|---|---|
| MR + C | 23.14 ± 21.61 | ||
| FDG—THbgd | 16.91 ± 16.49 | 0.85 | 0.58–0.73 |
| FDG—TH2.5 | 28.74 ± 26.22 | 0.88 | 0.73–0.94 |
| FDG—TH40% | 14.16 ± 10.61 | 0.63 | 0.25–0.82 |
| TATE—THbgd | 21.32 ± 19.72 | 0.95 | 0.91–0.99 |
| TATE—TH2.5 | 19.08 ± 23.26 | 0.91 | 0.81–0.95 |
| TATE—TH40% | 19.37 ± 18.52 | 0.93 | 0.83–0.97 |
ICC interclass correlation coefficient, CI confidence interval, FDG 18F-FDG, TATE 68Ga-DOTATATE.
Discussion
This study demonstrated that 68Ga-DOTATATE and 18F-FDG PET/CT have comparable performance in the detection of primary tumors and lymph node metastases. 68Ga-DOTATATE PET/CT demonstrated an equivalent number of lung metastases, revealing a greater number of bone metastases than 18F-FDG PET/CT. However, 68Ga-DOTATATE PET/CT was unable to detect liver metastases accurately. In 35/42 patients, 68Ga-DOTATATE and 18F-FDG PET/CT yielded consistent overall staging. In 6/42 patients, the overall staging on 18F-FDG PET/CT was corrected using 68Ga-DOTATATE PET/CT. Furthermore, tumor volumes defined by 68Ga-DOTATATE PET using various thresholds demonstrated greater concordance with MRI than 18F-FDG PET. As a result, 68Ga-DOTATATE PET/CT demonstrates significant clinical potential as an innovative imaging technique for evaluating NPC.
Compared to previous results14, this study demonstrated that primary tumors and lymph node metastases showed significantly higher uptake of 18F-FDG than 68Ga-DOTATATE. This may result from variations among the distinct small study cohorts. The uptake of the two radiotracers demonstrated a strong correlation despite differing imaging principles in paired double-positive primary tumors and lymph node metastases. The metabolic activity of a tumor serves as an indicator of its aggressiveness. The aggressiveness of certain cancers is positively correlated with SSTR expression22,23. This suggests that 68Ga-DOTATATE imaging could be useful in predicting the aggressiveness of NPC. In one of thirty-seven newly diagnosed patients, the uptake of 68Ga-DOTATATE was significant. However, MRI and 18F-FDG PET/CT could not identify the primary tumor. This suggests that 68Ga-DOTATATE PET/CT may demonstrate improved efficacy in identifying early mucosal lesions compared to traditional techniques. The efficacy of 18F-FDG PET/CT in distinguishing localized residual or recurrent disease from inflammation following NPC radiotherapy remains a subject of debate24. However, 68Ga-DOTATATE PET/CT was not more accurate than 18F-FDG imaging in the relapsed cases. The limited number of relapsed cases necessitates further investigation into detecting localized recurrence of NPC using 68Ga-DOTATATE PET/CT 18F-FDG, as a nonspecific tumor radiotracer, shows significant uptake in inflammatory lesions4. In this study, 18 lymph nodes with inflammatory or reactive hyperplasia displayed aberrant 18F-FDG uptake, whereas only 4 were false-positive on 68Ga-DOTATATE PET/CT. The N-staging of four patients was revised using 68Ga-DOTATATE PET/CT. Therefore, 68Ga-DOTATATE PET/CT may have higher specificity than 18F-FDG PET/CT in detecting lymph node metastases in patients with nasopharyngeal carcinoma.
In assessing distant metastases, lung, and bone metastases demonstrated a higher absorption of 18F-FDG compared to 68Ga-DOTATATE, whereas liver metastases demonstrated the inverse trend. The limitation of 68Ga-DOTATATE PET in identifying liver metastases arises from its inability to detect lesions with tracer uptake comparable to that of normal liver tissue, where SSTR2 expression is significantly elevated, thus decreasing the detection rate of liver metastases. 68Ga-DOTATATE PET/CT detected more bone metastases than 18F-FDG PET/CT. However, one bone metastasis (bone abnormality not shown on CT) was not detected by 68Ga-DOTATATE PET/CT, suggesting that it may not be sufficiently sensitive for detecting early bone metastases compared with 18F-FDG PET/CT 18F-FDG PET/CT was not sensitive enough to identify osteogenic metastases25. This study found that bone metastases lacking 18F-FDG positive uptake showed osteosclerosis on CT imaging. All demonstrated positive radiotracer uptake on 68Ga-DOTATATE PET/CT. One patient experienced a benefit from 68Ga-DOTATATE PET/CT, which corrected the M-staging of 18F-FDG, leading to appropriate treatment. While 68Ga-DOTATATE PET/CT showed high accuracy in identifying osteoblastic metastases of NPC in our study, cases of false-positive results were observed under specific conditions. Uptake of 68Ga-DOTATATE is observable in osteoblastic bone diseases due to the expression of somatostatin receptor type 2 by osteoblasts26. Degenerative bone disease, fractures, fibrous dysplasia, and vertebral hemangiomas all show 68Ga-DOTATATE uptake27–29. Integration with other imaging findings, such as CT/MRI characteristics and clinical data during imaging, is essential for differentiating between benign and malignant lesions rather than relying solely on 68Ga-DOTATATE uptake levels.
The precise measurement of tumor volume is essential for effective radiotherapy planning. The significant absorption of 18F-FDG by adjacent normal tissue complicates the evaluation of tumor extent using 18F-FDG PET/CT30. The lack of a unified segmentation method for lesions and the selection of SUV thresholds also led to low credibility in tumor volume delineated by 18F-FDG PET31. The study demonstrated that 68Ga-DOTATATE PET/CT exceeded 18F-FDG in assessing skull base bone, intracranial invasion, and T staging. This occurs due to the limited absorption of 68Ga-DOTATATE by the normal tissues surrounding the head and neck. This also functions as a prerequisite for precisely defining the biological target area in radiotherapy. In this study, the tumor volume measured by alternative threshold techniques was less than that obtained from MRI, except for 18F-FDG PET using TH2.5. This may result from interference caused by peritumoral edema and inflammation, which can lead to an overestimation of the tumor’s extent on MRI32. The tumor volume segmented using TH2.5 on 18F-FDG PET is relatively large. This is mainly because it is difficult to fully distinguish the boundary between the primary tumor and the adjacent non-tumor tissues with this threshold method. Compared with 18F-FDG PET, the tumor volumes measured by these three thresholds on 68Ga-DOTATATE PET showed smaller changes and were more consistent with MRI. The application of THbgd as a threshold method on 68Ga-DOTATATE PET for tumor volume assessment demonstrated the highest consistency with MRI results. Previous studies indicate that the THbgd exceeds other fixed threshold methods in tumor volume delineation21,33,34. The investigators observed that the 18F-FDG PET/CT using THbgd demonstrated a significantly higher detection rate of recurrent and metastatic lesions than TH2.5 and TH40%21. Therefore, it was speculated that 68Ga-DOTATATE PET/CT is a reliable supplement to MRI for helping with tumor volume delineation in NPC. Recently, Beichel et al.35 demonstrated advantages in 18F-FDG PET scans of head and neck tumors using a highly automated optimal surface segmentation method for uptake volume segmentation. This semiautomated segmentation approach, which integrates graph-based optimization with the just-enough-interaction principle, can adapt to metabolic characteristics of different tracers and has the potential to be further explored as a lesion segmentation method for 68Ga-DOTATATE PET scans in the future.
The limited number of participants represents a significant limitation of this study. Larger population sizes are required for prospective studies. This study employed multimodality imaging and follow-up examinations to identify most metastases, as collecting histopathologic results for all metastases was not clinically feasible. Therefore, further pathological evidence of metastases is required to confirm the diagnostic accuracy of 68Ga-DOTATATE PET/CT. Furthermore, visual bias affects tumor volumes on MRI, which are defined by visual assessment. In the future, it is necessary to enhance the study methodology to provide a more detailed analysis of the efficacy of 68Ga-DOTATATE PET/CT in NPC.
Conclusion
In conclusion, the 68Ga-DOTATATE PET/CT exhibited great clinical utility in evaluating NPC diagnosis and staging. In identifying bone metastases, lymph node metastases, skull base invasion, and intracranial involvement, 68Ga-DOTATATE PET/CT may demonstrate improved efficacy compared to 18F-FDG. The 68Ga-DOTATATE PET/CT can obtain accurate tumor volume delineation. Using THbgd as the ideal threshold technique could be a valuable addition to MRI for NPC target volume delineation. Further research is necessary to determine the clinical usefulness of 68Ga-DOTATATE PET/CT in radiotherapy planning for NPC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the members of Department of Nuclear Medicine, The Affiliated Hospital, Southwest Medical University and Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province for their technical guidance, cooperation and assistance in completing this article.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by H.Y.D. and L.J. The first draft of the manuscript was written by H.Y.D. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Luzhou Science and Technology Program (2024JYJ054), National Natural Science Foundation of China (U20A20384), Health Commission of Sichuan Province (21ZD005), Science and Technology Major Project of Gansu Province (23ZDFA014), and Isotope and Drug Innovation Fund of National Engineering Research Center for Isotopes and Drugs (TWSCX-2023-CXJJ-3-1).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Review Board of the Affiliated Hospital of Southwest Medical University (2020035). All procedures involving human participants were performed in accordance with the ethical standards of the institutional committee, as well as the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study does not contain any animal experiments. Informed consent was obtained from all participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Haoyuan Ding and Juan Liang.
These authors jointly supervised this work: Ya Liu and Yue Chen.
Contributor Information
Ya Liu, Email: 1935221414@qq.com.
Yue Chen, Email: chenyue5523@126.com.
References
- 1.Chen, Y. P. et al. Nasopharyngeal carcinoma. Lancet394, 64–80 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Au, K. H. et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study). Oral Oncol.77, 16–21 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Lee, S. H. et al. Diagnostic value of only 18F-fluorodeocyglucose positron emission tomography/computed tomography-positive lymph nodes in head and neck squamous cell carcinoma. Otolaryngol. Head Neck Surg.147, 692–698 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Fei, Z. et al. Metabolic tumor volume and conformal radiotherapy based on prognostic PET/CT for treatment of nasopharyngeal carcinoma. Medicine98, e16327 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Bosch, S. et al. 18)F-FDG-PET/CT-based treatment planning for definitive (chemo)radiotherapy in patients with head and neck squamous cell carcinoma improves regional control and survival. Radiother Oncol.142, 107–114 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Zaidi, H. & El Naqa, I. PET-guided delineation of radiation therapy treatment volumes: a survey of image segmentation techniques. Eur. J. Nucl. Med. Mol. Imaging. 37, 2165–2187 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Riegel, A. C. et al. Variability of gross tumor volume delineation in head-and-neck cancer using CT and PET/CT fusion. Int. J. Radiat. Oncol. Biol. Phys.65, 726–732 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Hong, R., Halama, J., Bova, D., Sethi, A. & Emami, B. Correlation of PET standard uptake value and CT window-level thresholds for target delineation in CT-based radiation treatment planning. Int. J. Radiat. Oncol. Biol. Phys.67, 720–726 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Heron, D. E. et al. Hybrid PET-CT simulation for radiation treatment planning in head-and-neck cancers: a brief technical report. Int. J. Radiat. Oncol. Biol. Phys.60, 1419–1424 (2004). [DOI] [PubMed]
- 11.Xu, M. et al. Genome sequencing analysis identifies Epstein-Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat. Genet.51, 1131–1136 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lechner, M. et al. Somatostatin receptor 2 expression in nasopharyngeal cancer is induced by epstein barr virus infection: impact on prognosis, imaging and therapy. Nat. Commun.12, 117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viswanathan, K. & Sadow, P. M. Somatostatin receptor 2 is highly sensitive and specific for Epstein-Barr virus-associated nasopharyngeal carcinoma. Hum. Pathol.117, 88–100 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao, L. et al. Somatostatin receptor imaging with [ (68)Ga]Ga-DOTATATE positron emission tomography/computed tomography (PET/CT) in patients with nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 49, 1360–1373 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Zheng, J. et al. A head-to-head comparison of [(68)Ga]Ga-DOTATATE and [(68)Ga]Ga-FAPI PET/CT in patients with nasopharyngeal carcinoma: a single-center, prospective study. Eur. J. Nucl. Med. Mol. Imaging. 51, 3472–3474 (2024). [DOI] [PubMed] [Google Scholar]
- 16.Liu, L. et al. The impact of total variation regularized expectation maximization reconstruction on (68)Ga-DOTA-TATE PET/CT images in patients with neuroendocrine tumor. Front. Med. (Lausanne). 9, 845806 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding, H. et al. Prospective comparison of (68)Ga-FAPI-04 and (18)F-FDG PET/CT for tumor staging in nasopharyngeal carcinoma. Front. Oncol.12, 1047010 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan, J. J. et al. Ninth version of the AJCC and UICC nasopharyngeal cancer TNM staging classification. JAMA Oncol.10, 1627–1635 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caudell, J. J. et al. NCCN guidelines® insights: head and neck cancers, version 1.2022. J. Natl. Compr. Cancer Netw.20, 224–234 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer. 45, 228–247 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Tian, Y. et al. Prognostic value of volume-based positron emission tomography/computed tomography in nasopharyngeal carcinoma patients after comprehensive therapy. Contrast Media Mol. Imaging2018, 1384281 (2018). [DOI] [PMC free article] [PubMed]
- 22.Provost, C. et al. 68Ga-DOTATOC and FDG PET imaging of preclinical neuroblastoma models. Anticancer Res.36, 4459–4466 (2016). [DOI] [PubMed] [Google Scholar]
- 23.de Sá, S. V. et al. Somatostatin receptor subtype 5 (SSTR5) mRNA expression is related to histopathological features of cell proliferation in insulinomas. Endocr. Relat. Cancer. 13, 69–78 (2006). [DOI] [PubMed] [Google Scholar]
- 24.OuYang, P. Y. et al. Benefit of [ (18)F] FDG PET/CT in the diagnosis and salvage treatment of recurrent nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 50, 881–891 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Zhang, Y. et al. Comparison of (18)F-NaF PET/CT and (18)F-FDG PET/CT for detection of skull-base invasion and osseous metastases in nasopharyngeal carcinoma. Contrast Media Mol. Imaging2018, 8271313 (2018). [DOI] [PMC free article] [PubMed]
- 26.Mackie, E. J., Trechsel, U. & Bruns, C. Somatostatin receptors are restricted to a subpopulation of osteoblast-like cells during endochondral bone formation. Development110, 1233–1239 (1990). [DOI] [PubMed] [Google Scholar]
- 27.Klinaki, I., Al-Nahhas, A., Soneji, N. & Win, Z. 68Ga DOTATATE PET/CT uptake in spinal lesions and MRI correlation on a patient with neuroendocrine tumor: potential pitfalls. Clin. Nucl. Med.38, e449–453 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Filizoglu, N., Bugdayci, O. & Ozguven, S. Sacral insufficiency fracture on 68Ga-DOTATATE PET/CT. Clin. Nucl. Med.46, e490–e491 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Papadakis, G. Z. et al. Fibrous dysplasia mimicking malignancy on 68Ga-DOTATATE PET/CT. Clin. Nucl. Med.42, 209–210 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King, A. D. et al. The impact of 18F-FDG PET/CT on assessment of nasopharyngeal carcinoma at diagnosis. Br. J. Radiol.81, 291–298 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Chen, Y. Z. et al. Evaluation of time-phase effect on 18F-FDG PET/CT delineation methods for treatment planning of nasopharyngeal carcinoma. Clin. Nucl. Med.41, 354–361 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Ng, S. H. et al. Clinical usefulness of 18F-FDG PET in nasopharyngeal carcinoma patients with questionable MRI findings for recurrence. J. Nucl. Med.45, 1669–1676 (2004). [PubMed] [Google Scholar]
- 33.Yu, W. et al. Y. GTV Spatial conformity between different delineation methods by 18FDG PET/CT and pathology in esophageal cancer. Radiother Oncol.93, 441–446 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Li, C. et al. Evaluation of 11C-choline PET/CT for T staging and tumor volume delineation in nasopharyngeal cancer patients in comparison to 18F-FDG PET/CT. Clin. Nucl. Med.48, 563–573 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Beichel, R. R. et al. Semiautomated segmentation of head and neck cancers in 18F-FDG PET scans: A just-enough-interaction approach. Med. Phys.43, 2948–2964 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.