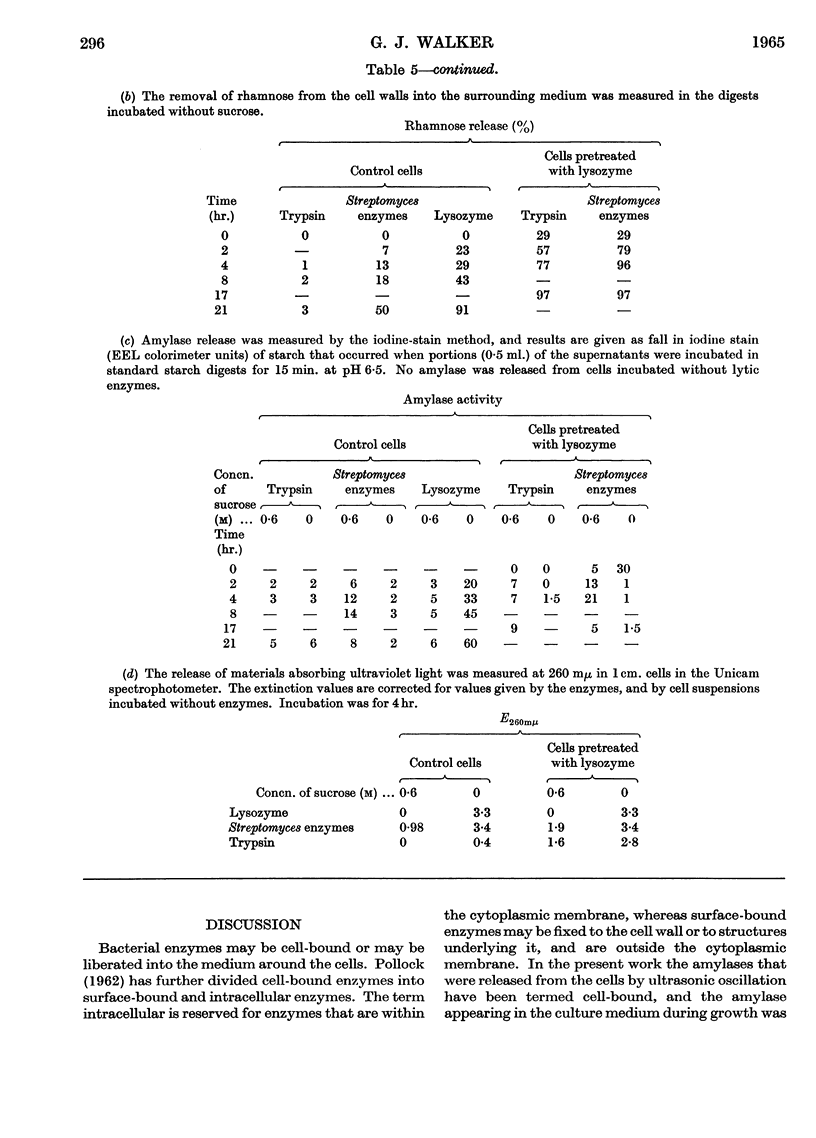
Abstract
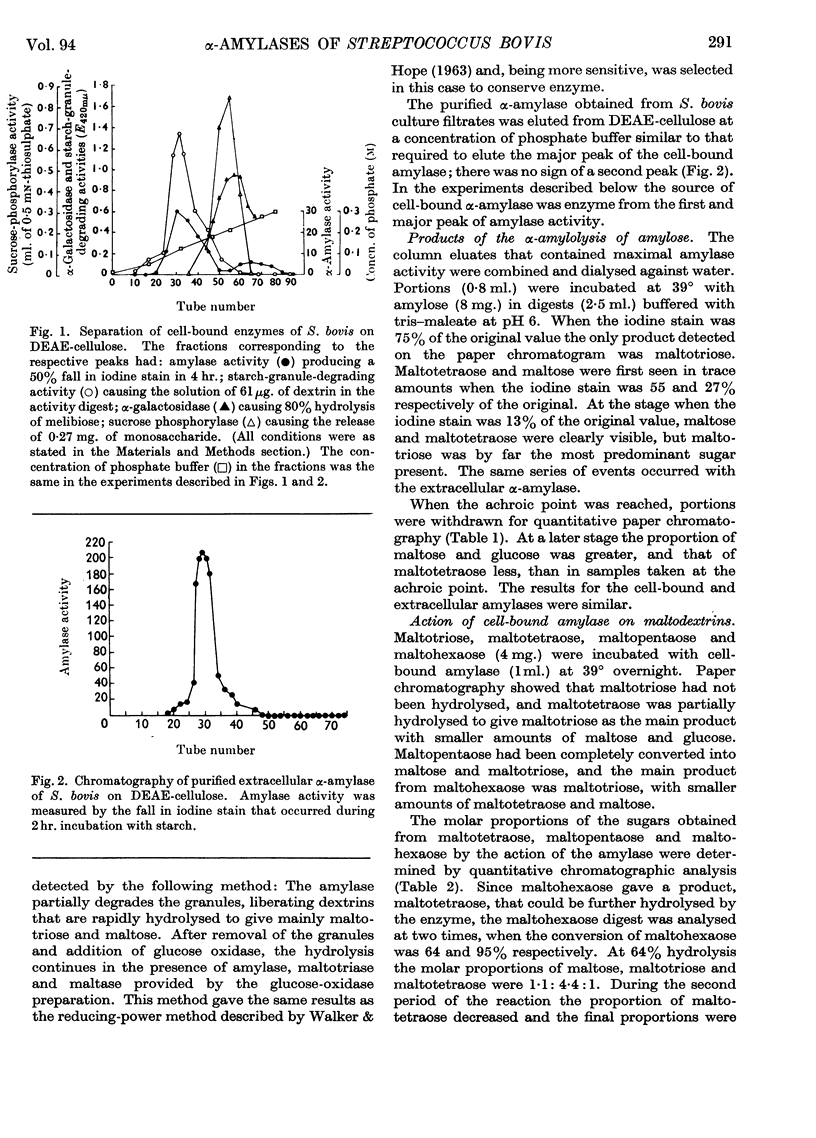
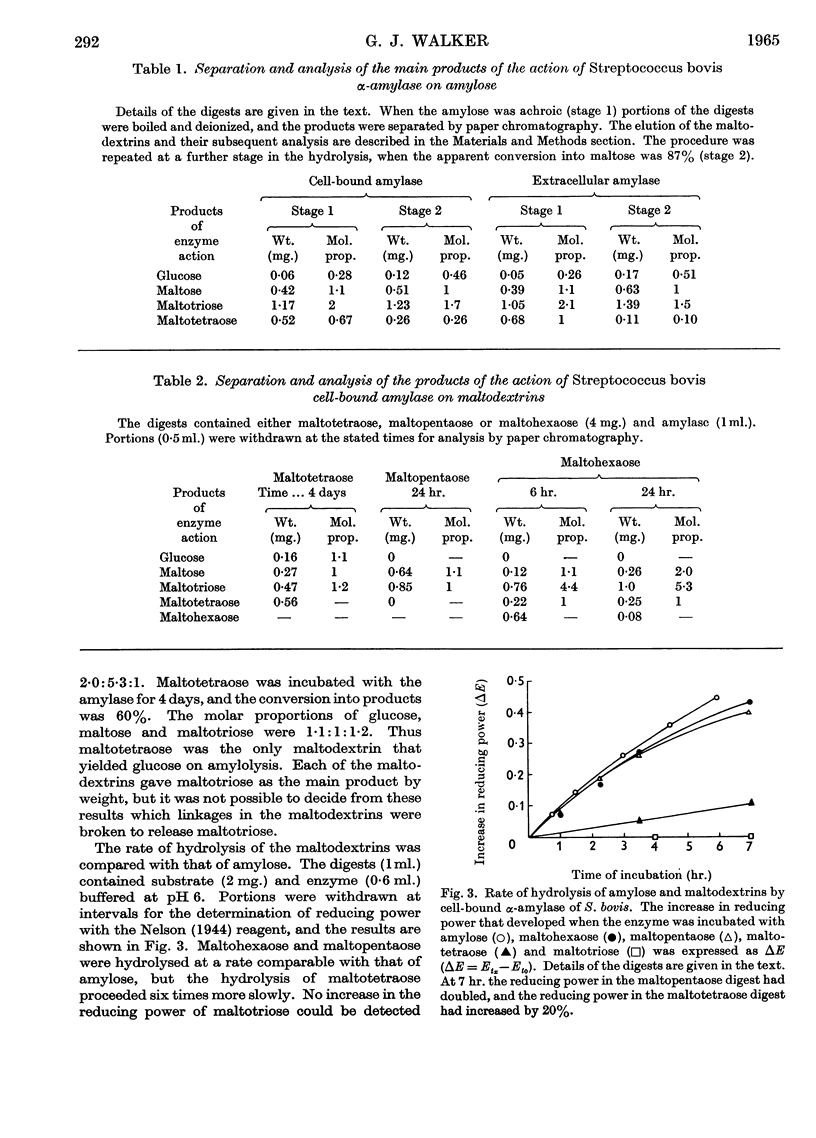
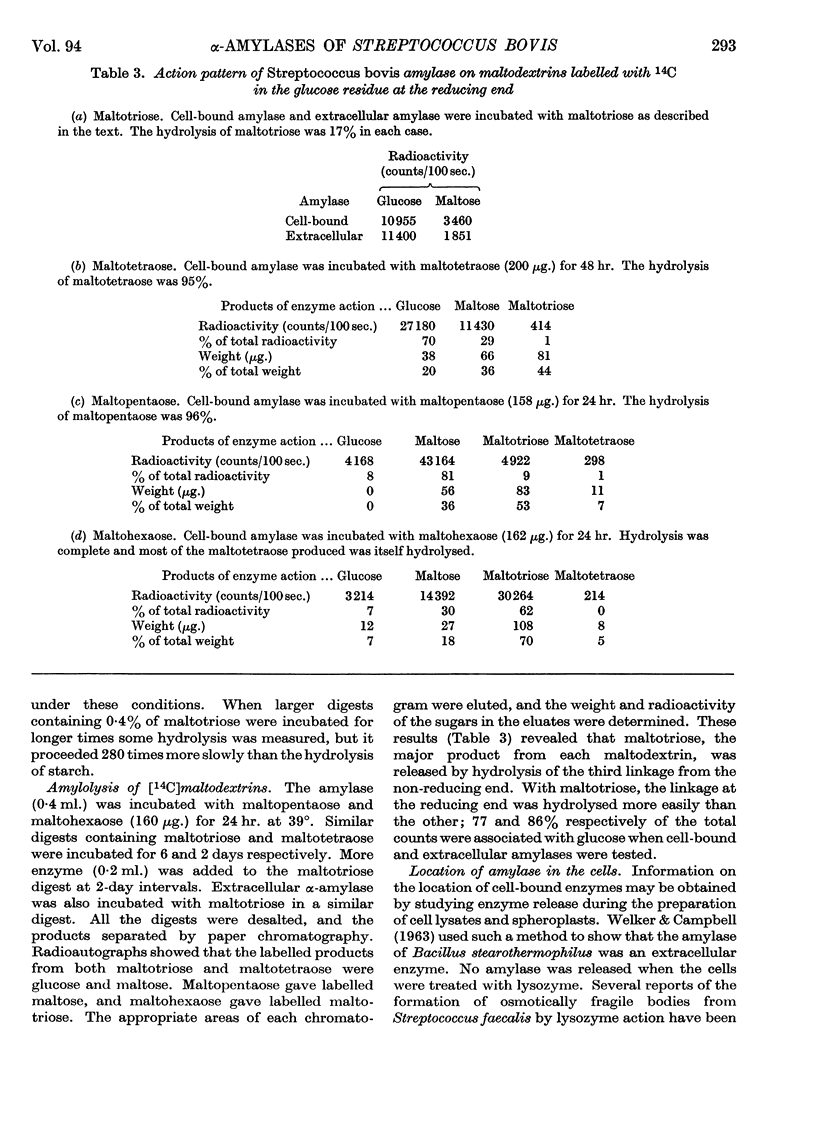
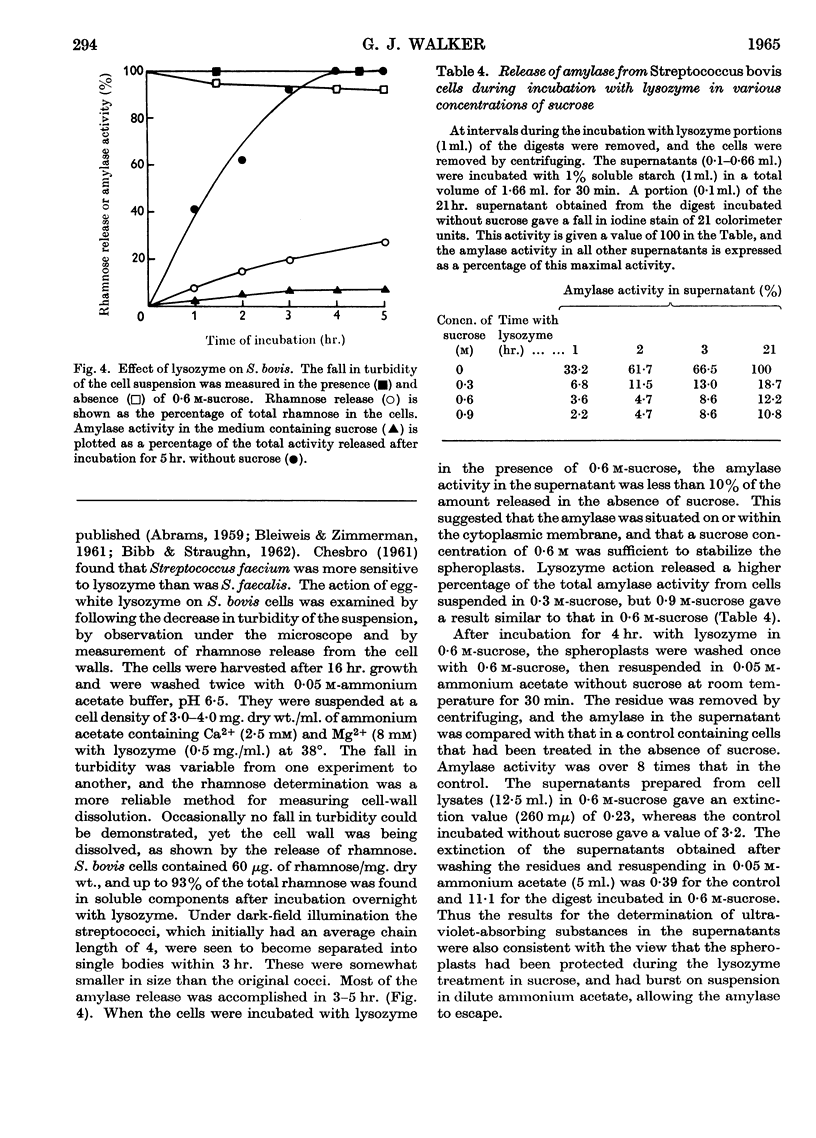
1. The cell-bound α-amylase of Streptococcus bovis has been isolated from other carbohydrases in the cell extract by chromatography on DEAE-cellulose. The enzyme has been compared with the extracellular α-amylase produced by this organism. 2. The two amylases had similar action patterns on amylose, the main product being maltotriose with smaller amounts of maltose and a little glucose. 3. The cell-bound amylase hydrolysed maltopentaose and maltohexaose at a similar rate to the hydrolysis of amylose. Maltotetraose was hydrolysed six times more slowly, and maltotriose 280 times more slowly, than amylose. 4. Studies with end-labelled maltodextrins revealed that the cell-bound α-amylase preferentially hydrolysed the third linkage from the non-reducing end, liberating maltotriose. The linkage at the reducing end of maltotriose was more easily hydrolysed than the other. 5. Egg-white lysozyme and the extracellular enzymes of Streptomyces albus lysed the cell walls of Streptococcus bovis, releasing amylase into the medium. In the presence of 0·6 m-sucrose 10% of the maximal amylase activity was released by lysozyme. Suspension of the spheroplasts in dilute buffer caused the rupture of the cytoplasmic membrane and the liberation of amylase. 6. A sensitive method for determining the ability of amylases to degrade starch granules is described.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A. Reversible metabolic swelling of bacterial protoplasts. J Biol Chem. 1959 Feb;234(2):383–388. [PubMed] [Google Scholar]
- BIBB W. R., STRAUGHN W. R. Formation of protoplasts from Streptococcus faecalis by lysozyme. J Bacteriol. 1962 Nov;84:1094–1098. doi: 10.1128/jb.84.5.1094-1098.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBSON P. N., MACPHERSON M. Amylases of Clostridium butyricum and a Streptococcus isolated from the rumen of the sheep. Biochem J. 1952 Dec;52(4):671–679. doi: 10.1042/bj0520671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- MCCARTY M. The lysis of group A hemolytic streptococci by extracellular enzymes of Streptomyces albus. I. Production and fractionation of the lytic enzymes. J Exp Med. 1952 Dec;96(6):555–568. doi: 10.1084/jem.96.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAZUR J. H., BUDOVICH T. Hydrolysis of amylotriose by crystalline salivary amylase. Science. 1955 May 13;121(3150):702–703. doi: 10.1126/science.121.3150.702. [DOI] [PubMed] [Google Scholar]
- ROBYT J., FRENCH D. Action pattern and specificity of an amylase from Bacillus subtilis. Arch Biochem Biophys. 1963 Mar;100:451–467. doi: 10.1016/0003-9861(63)90112-7. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WALKER G. J. A TRANSGLUCOSYLASE OF STREPTOCOCCUS BOVIS. Biochem J. 1965 Feb;94:299–308. doi: 10.1042/bj0940299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER G. J., HOPE P. M. The action of some alpha-amylases on starch granules. Biochem J. 1963 Mar;86:452–462. doi: 10.1042/bj0860452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. EFFECT OF CARBON SOURCES ON FORMATION OF ALPHA-AMYLASE BY BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1963 Oct;86:681–686. doi: 10.1128/jb.86.4.681-686.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


