Abstract
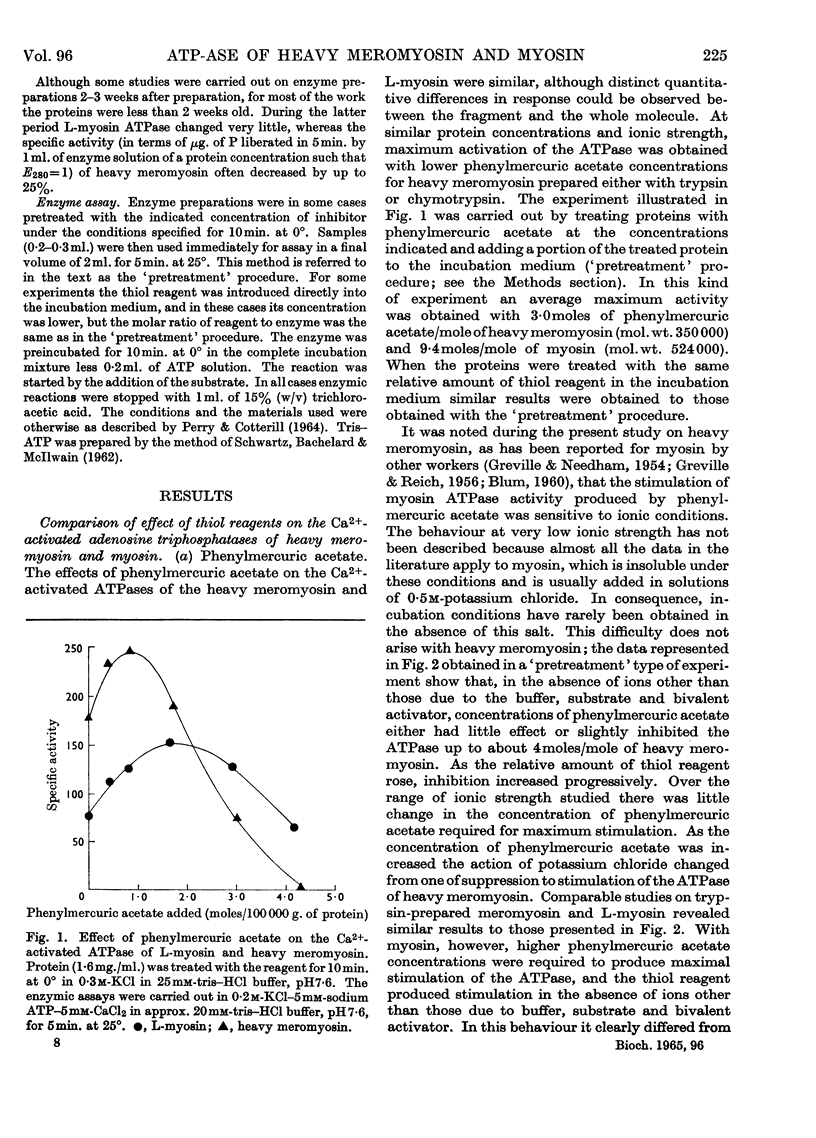
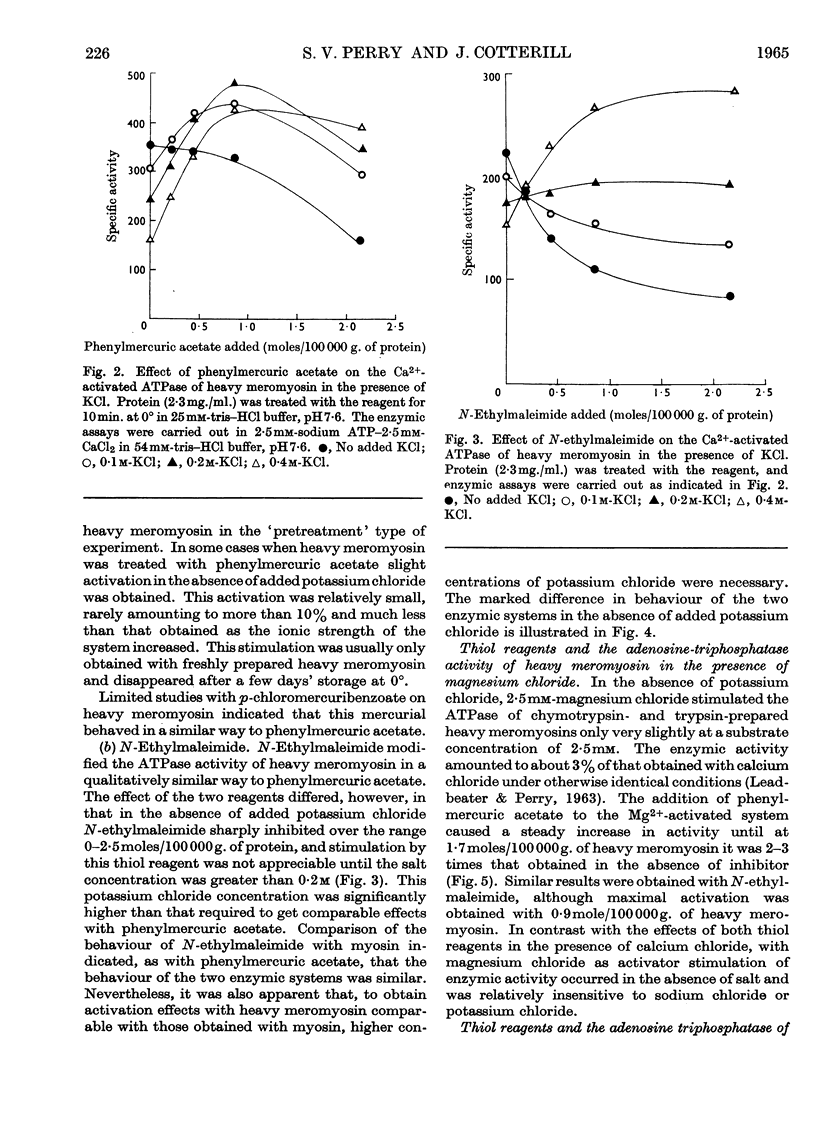
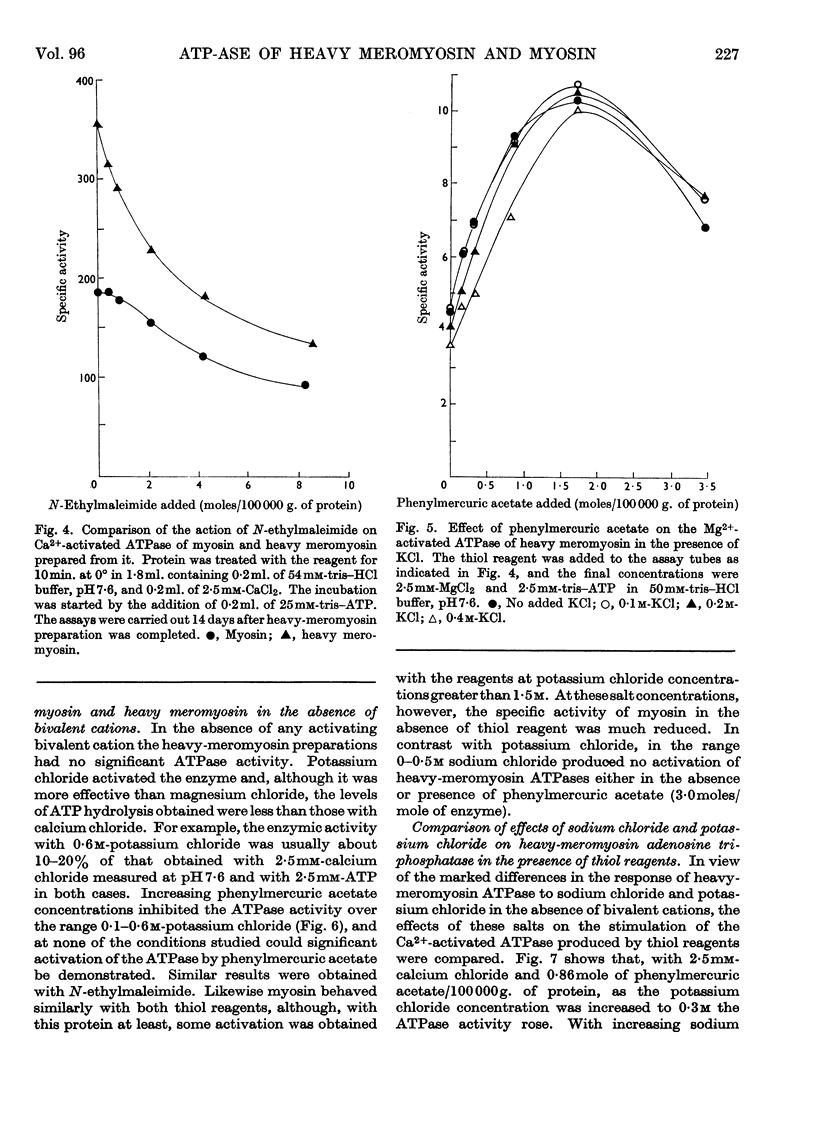
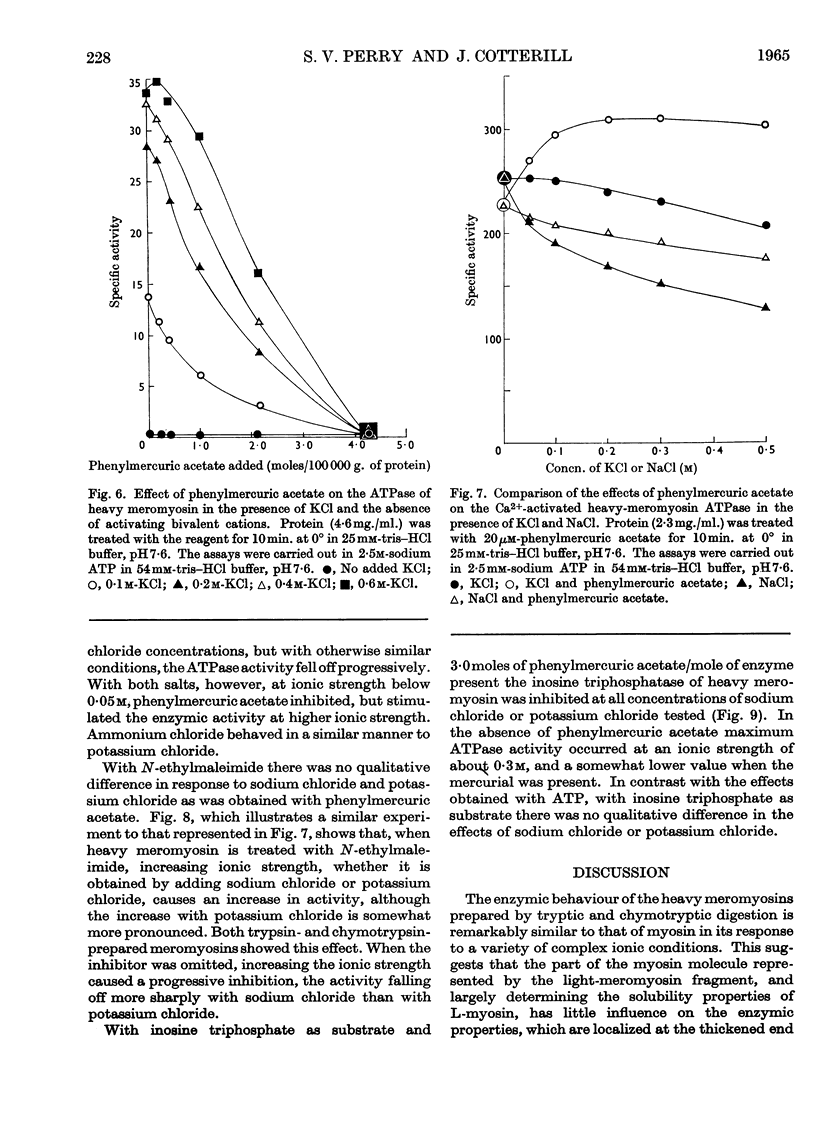
1. The Ca2+-activated adenosine triphosphatase of heavy meromyosin is maximally stimulated by lower relative molar concentrations of phenylmercuric acetate than are required with myosin. 2. Stimulation of the Ca2+-activated adenosine triphosphatase of both heavy meromyosin and myosin by thiol reagents is markedly affected by ionic strength, the effects being greater with the former than with the latter. In particular, N-ethylmaleimide strongly inhibits the Ca2+-activated adenosine triphosphatase of heavy meromyosin at ionic strength below about 0·2. 3. The precise behaviour of the thiol reagents at low ionic strength is slightly modified by the age of the heavy meromyosin and myosin preparations. 4. Stimulation of the Mg2+-activated adenosine triphosphatase of heavy meromyosin by thiol reagents is relatively insensitive to ionic strength. 5. The adenosine triphosphatases of heavy meromyosin and myosin activated by potassium chloride in the absence of bivalent activators are inhibited by thiol reagents over the range of ionic strength at which stimulation occurs in the presence of calcium chloride as activator. 6. The modifying effects of potassium chloride and sodium chloride are qualitatively different when heavy-meromyosin adenosine triphosphatase is stimulated with phenylmercuric acetate. No such difference is observed when the enzyme is stimulated with N-ethylmaleimide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUM J. J., FELAUER E. Effect of dinitrophenol on the interaction between myosin and nucleotides. Arch Biochem Biophys. 1959 Apr;81(2):285–299. doi: 10.1016/0003-9861(59)90206-1. [DOI] [PubMed] [Google Scholar]
- BLUM J. J. Interaction between myosin and its substrates. Arch Biochem Biophys. 1960 Mar;87:104–119. doi: 10.1016/0003-9861(60)90130-2. [DOI] [PubMed] [Google Scholar]
- GERGELY J. Studies on myosin-adenosinetriphosphatase. J Biol Chem. 1953 Feb;200(2):543–550. [PubMed] [Google Scholar]
- GREVILLE G. D., NEEDHAM D. M. Effect of 2:4-dinitrophenol and phenylmercuric acetate on enzymic activity of myosin. Biochim Biophys Acta. 1955 Feb;16(2):284–285. doi: 10.1016/0006-3002(55)90216-x. [DOI] [PubMed] [Google Scholar]
- GREVILLE G. D., REICH E. Effects of 2:4-dinitrophenol and other agents on the nucleoside triphosphatase activities of L-myosin. Biochim Biophys Acta. 1956 May;20(2):440–442. doi: 10.1016/0006-3002(56)90334-1. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN J. M. The stimulation of transphosphorylation by alkali-metal ions. Biochem J. 1960 May;75:269–274. doi: 10.1042/bj0750269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIHALYI E., SZENT-GYORGYI A. G. Trypsin digestion of muscle proteins. I. Ultracentrifugal analysis of the process. J Biol Chem. 1953 Mar;201(1):189–196. [PubMed] [Google Scholar]
- MUELLER H., PERRY S. V. The degradation of heavy meromyosin by trypsin. Biochem J. 1962 Dec;85:431–439. doi: 10.1042/bj0850431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V. The ATP-ase activity of isolated myofibrils. Biochem J. 1950 Sep;47(3):xxxviii–xxxviii. [PubMed] [Google Scholar]
- PERRY S. V. The adenosinetriphosphatase activity of myofibrils isolated from skeletal muscle. Biochem J. 1951 Mar;48(3):257–265. doi: 10.1042/bj0480257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cotterill J. The action of thiol inhibitors on the interaction of F-actin and heavy meromyosin. Biochem J. 1964 Sep;92(3):603–608. doi: 10.1042/bj0920603. [DOI] [PMC free article] [PubMed] [Google Scholar]


