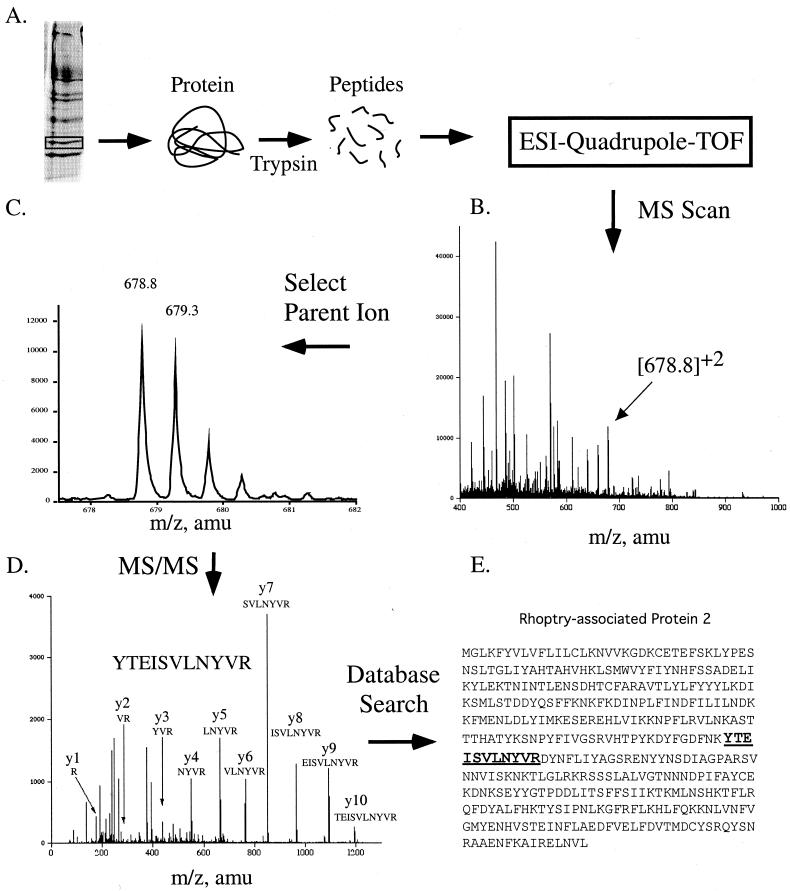
FIG. 8.
Protein identification by MS/MS. (A) Protein from P. falciparum was resolved on a one-dimensional polyacrylamide gel, excised, and in-gel digested with trypsin. The resulting peptides were ionized by electrospray and analyzed by a Quadrupole-TOF mass spectrometer. (B) The MS spectrum produced was scanned, and a parent ion of 678.8 was selected for fragmentation. (C) Enlargement of the parent ion peak at 678 shown in panel B. The multiplet of peaks is due to the contribution in mass from the naturally occurring isotope 13C. A mass difference between the peaks of 0.5 Da indicates that the peptide is doubly charged. (D) MS/MS scan of the 678 parent ion and analysis of the daughter ions produced. All y-ions (except for y-11) produced from fragmentation of the peptide are shown. (E) Identification of rhoptry-associated protein-2 using BioAnalyst software (Applied Biosystems, Foster City, Calif.).