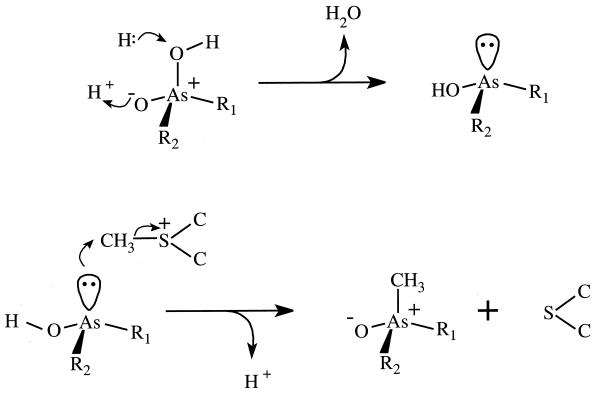
FIG. 2.
Typical reactions of the Challenger mechanism. The top line indicates a mechanism for the reduction, As(V) → As(III), resulting in an unshared pair of electrons on As. Structures are as follows: R1 = R2 = OH, arsenate; R1 = CH3, R2 = OH, methylarsonate; R1 = R2 = CH3, dimethylarsinate. For reduction of trimethylarsine oxide to trimethylarsine, the process is a little different. Following proton addition, the structure H-O-As+(CH3)3 reacts with hydride ion leading to elimination of H2O. The bottom line indicates the methylation of an As(III) structure with SAM [shown in abbreviated form as CH3-S+-(C)2]. A proton is released and SAM is converted to S-adenosylhomocysteine [abbreviated form, S-(C)2].