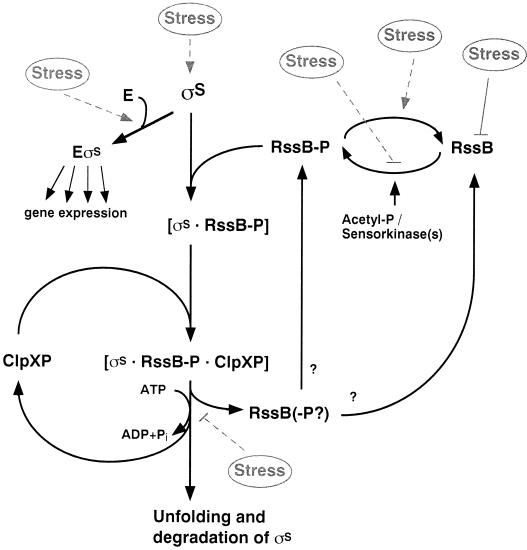
FIG. 4.
Role of RssB-ClpXP and putative signal input in the σS recognition and degradation pathway. The response regulator RssB is an essential, specific, and direct σS recognition factor. RssB delivers σS to the ClpXP protease, where σS is unfolded and completely degraded whereas RssB is released. σS binding requires RssB phosphorylation, but it is unclear whether the catalytic cycle of RssB involves obligatory dephosphorylation during release and subsequent rephosphorylation. Stress signals may affect (i) the phosphorylation of RssB and therefore RssB-σS complex formation; (ii) the cellular level of RssB (which in growing cells is rate limiting for σS proteolysis); (iii) the synthesis of σS such that RssB becomes titrated on σS overproduction; (iv) σS association with RNA polymerase core enzyme, which protects against binding by RssB; and (v) the function of the ClpXP protease itself (see the text for details). However, the molecular details of the stress signal input pathways involved are still largely unknown.