Abstract
Neuroendocrine neoplasms (NENs) are a group of highly heterogeneous neoplasms originating from neuroendocrine cells with a gradually increased incidence. Metabolic change is one of the recognized markers of tumor progression, which has been extensively and systematically studied in other malignant tumors. However, metabolic change in NENs has been relatively poorly studied, and systematic reviews are lacking. We reviewed the relationship between metabolic changes and NENs from the aspects of glucose metabolism, lipid metabolism, metabolic syndrome, amino acid metabolism and metabolomics, and discussed the potential therapeutic strategies of metabolic changes for NENs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-025-05656-2.
Keywords: Neuroendocrine neoplasm, Metabolic changes, Metabolite
Introduction
Neuroendocrine neoplasms (NENs) are a highly heterogeneous group of tumors that originate from peptidergic neurons and neuroendocrine cells found throughout the body, including the gastrointestinal tract, lungs, pancreas, and other less common locations like the adrenal glands and thymus [1]. Among these, gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) are the most prevalent [2, 3]. Gastric NENs are relatively uncommon and can be either sporadic or associated with hereditary syndromes like Multiple Endocrine Neoplasia Type 1 (MEN1) [4]. Small intestinal NENs, particularly those arising in the ileum, are among the most frequent types of gastrointestinal NENs. Rectal NENs are usually small and well-differentiated, often discovered incidentally during routine colonoscopies. Pancreatic NENs (pNENs) can be functioning or non-functioning, depending on whether they secrete hormones that cause clinical symptoms. Common types include insulinomas, gastrinomas, and glucagonomas, each named for the hormone they overproduce [2, 5].
NENs was previously considered to be a rare disease, but the incidence of NENs has increased in recent decades, due to heightened health awareness, the expanded use of endoscopy, and advancements in diagnostic imaging techniques within nuclear medicine. According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database, the incidence rate of NENs has been steadily climbing. The SEER database reveals that the current annual incidence rate stands at 8.4 per 100,000 individuals, marking an increase of nearly 8 times from 1973 (1.09 per 100 000). The increase occurred across all sites, stages, and grades. This growth rate significantly outpaces that of other malignancies, highlighting NENs as a grave threat to human life and health [6–9].
Neuroendocrine neoplasms (NENs) can be categorized into functional NENs and non-functional NENs. Functional NENs are characterized by their secretion of peptides or amine hormones, leading to distinct clinical symptoms. In contrast, non-functional NENs often have a subtle onset and lack distinctive clinical signs or symptoms. This can result in delayed detection and misdiagnosis, with an average diagnostic delay of approximately 5 to 7 years [10]. About 30% of GEP-NENs are hormonally active and can cause specific clinical syndromes. The clinical presentation mainly depends on the primary site of the tumor and its functionality [11]. Neuroendocrine paraneoplastic syndromes (PNS) are a series of clinical symptoms and signs attributed to metabolic disorders caused by functional NENs secreting functional peptides, hormones, cytokines, peptides, or growth factors [12, 13]. Functional NENs mainly include carcinoid tumors, followed by insulinoma, pancreatic polypeptide tumor, gastrinoma, VIPoma, glucagonoma, somatostatinoma, gastrinoma, and other rare tumors [14]. The carcinoid syndrome is characterized by recurrent flushing and secretory diarrhea caused by high levels of circulating serotonin and other vasoactive peptides [15]. Several metabolites and peptides produced by NENs are involved in cell signaling and promote tumor proliferation in an autocrine and paracrine manner. Ishizuka et al. showed that serotonin can activate the 5HT1A/1B receptor on the surface of cultured human pancreatic neuroendocrine tumor cells, whereas serotonin receptor antagonists can inhibit cell proliferation, suggesting an autocrine effect of serotonin on NE tumor cells [16]. Ghrelin is significantly associated with the regulation of fat metabolism, and ghrelin replacement therapy may alleviate weight loss after gastrectom [17]. Hormonal excess and tumor progression seriously affect the quality of life and prognosis of patients with NENs, but the specific molecular mechanism has not been fully elucidated.
NENs can be classified into well-differentiated G1, G2, G3 neuroendocrine tumors (NETs) and poorly-differentiated neuroendocrine carcinoma (NEC) according to Ki67 index, mitotic count and degree of differentiation [18, 19]. The pathological grade of NENs is closely related to the choice of treatment regimens and the prognosis of patients. The molecular and genetic features of NENs are highly heterogeneous and vary according to primary site, degree of differentiation, and proliferation grade [20]. NEC is most commonly characterized by mutations in key driver genes such as TP53 and RB1 [21]. In contrast, the molecular signature of NET lacks mutations in key oncogenes. It is characterized by genetic pathways related to chromatin remodeling and telomere maintenance (such as MEN1, ATRX, DAXX and ARID1A genes), PI3K/Akt/mTOR signaling pathways (such as PTEN and TSC2 genes) and VEGF pathways, and genetic and epigenetic alterations associated with cell cycle regulation, such as the CDKN1A and CDKN1B genes [22]. And TP53 mutations can also be observed in G3 NET [23]. NENs display distinct molecular alterations in different primary anatomical sites [24]. In the study by Yachida et al., TP53, KRAS, RB1, CCNE1, CDKN2A, APC, and MYC, in order of frequency, were confirmed to be the most mutated genes in digestive NEC [25]. RB1 mutations were more frequently found in pancreatic NEC [26], whereas Notch family gene changes were found exclusively in non-pancreatic NEC [25]. BRAF mutations were more common in colonic NEC than other sites [26], and alterations of the WNT/β-catenin pathways were found in more than 50% of gastric NEC [27]. It has shown that KRAS/TP53 co-mutant tumors have significantly reduced expression of genes related to glycolysis, whereas expression of genes related to lipid metabolism have increased [28]. The RB-E2F pathway plays a key role in regulating glucose metabolism, and loss of RB function leads to increased glycolysis [29].
Generally, well-differentiated neuroendocrine neoplasms (NENs) exhibit indolent growth in the early stage, while poorly differentiated NENs demonstrate malignant and invasive growth. However, NENs show the characteristics of “small lesions and large metastases”, so that even well-differentiated NENs can develop distant metastasis, complicating their clinical management and prognosis [30–32]. Once metastasis occurs, the availability of effective treatments is limited, and the prognosis becomes extremely poor, with the 5-year survival rate dramatically decreasing from 78 to 93% to 19–38% ADDIN EN.CITE [33]. Surgery remains the optimal treatment for localized NENs, offering the best chance for a complete resection and potential cure. In contrast, for patients with advanced metastatic NENs, a multifaceted approach is often necessary. The systemic therapies include somatostatin analogue therapy, chemotherapy, targeted therapies, interventional procedures, and radionuclide therapy, tailored to the individual’s specific condition and the extent of the disease [34, 35]. With the incidence of neuroendocrine neoplasms (NENs) on the rise, the challenges in diagnosing and treating these tumors are becoming increasingly apparent. Despite this, basic and clinical research about NENs has not kept pace with that of other malignant tumors. It is imperative that we adopt a novel approach to understanding the mechanisms of neuroendocrine neoplasm progression, identifying new therapeutic targets and developing innovative anti-tumor drugs to combat these challenging malignancies.
Metabolic change is widely acknowledged as a hallmark of tumor progression, particularly in the context of tumor metastasis [36]. Metabolic change refers to the process by which tumor cells modify their metabolic pathways of cellular anabolism and catabolism in order to adapt to the requirements of sustained proliferation and survival within a specific tissue environment, so as to provide sufficient energy and substances for tumor proliferation and metastasis, and alter cell differentiation and tumor microenvironment, which is critical for the long-term survival of the tumor [37–39]. The study of tumor metabolic change has a rich history, dating back nearly a century to Otto Warburg’s pivotal discovery in 1923. Warburg observed that, even in the presence of ample oxygen, tumor cells preferentially metabolize glucose through glycolysis to produce lactate, rather than the more efficient oxidative phosphorylation, known as the Warburg effect [40]. An increasing body of research underscores the pivotal role of metabolic changes in the development of diverse tumors. On the one hand, the metastatic progression of tumors necessitates a substantial energy supply, which is derived from altered energy metabolism. On the other hand, specific metabolites have been implicated as oncometabolite, highlighting the multifaceted impact of metabolic changes on tumor progression and metastasis [41–45].
To gain a systematic understanding of the role of metabolic changes in the development of tumor, Pavlova and Thompson have summarized and refined ten major characteristics of tumor metabolism: (1) dysregulated uptake of glucose and amino acids; (2) utilization of central carbon metabolism to support biosynthesis; (3) adoption of opportunistic nutrient acquisition strategies; (4) increased reliance on electron receptors such as NAD+; (5) heightened demand for oxidative stress protection mechanisms; (6) an augmented need for nitrogen; (7) diversity and heterogeneity in tumor metabolism; (8) metabolite-driven changes in gene regulation; (9) complex interactions between metabolism and the tumor microenvironment; (10) systemic metabolic regulation of cancerous lesions within the organism [46, 47]. Metabolic change has been systematically studied in the progression of various cancers, but research on the progression of NENs and metabolic change is relatively few and lack of systematic review. This paper aims to collate and summarize the research progress on the metabolism of NENs in recent years, and to chart a course for future research directions in the field of metabolic changes and NEN development. The figures are created with BioRender.
Results
Changes of glucose metabolism and NENs
Pathoglycemia and NENs
Tumor cells exhibit a pronounced reliance on glucose for processes such as glycolysis, glycogen metabolism, and gluconeogenesis, which is more pronounced than that of their normal counterparts [48]. Several pieces evidences have shown that hyperglycaemia promotes the invasiveness of breast cancer [49], lung cancer [50] and pancreatic cancer [51], whereas hypoglycaemia synergistically with metformin inhibits tumour growth and metabolic changes by regulating the PP2A- GSK3β-MCL-1 axis [52]. The pancreas plays a crucial role in regulating glucose metabolism, which in turn may have an impact on pancreatic tumor cells. A retrospective clinical study based on 335 patients with pancreatic NENs (pNENs) found that patients with hyperglycemia, defined by baseline fasting glucose concentrations of 5.6 mmol/L or higher, showed a higher proportion of lymph node metastasis and distant metastasis. Furthermore, multivariate analysis identified hyperglycemia as an independent risk factor for poorer overall survival [53].
In a study examining preoperative blood glucose and glycated hemoglobin levels of 417 patients who underwent surgical resection for pancreatic NENs, 88 had a history of diabetes mellitus, and 30 exhibited preoperative blood glucose abnormalities despite no prior diabetes diagnosis. The analysis indicated no significant differences in pathological characteristics or prognosis between patients with and without diabetes. However, patients with preoperative glucose abnormalities showed a higher rate of metastasis, a higher proportion of vascular, perineural and lymphovascular involvement, and diminished overall and recurrence-free survival rates. Multifactorial analysis identified preoperative glucose abnormalities as an independent risk factor for recurrence-free survival, lymph node metastasis, and distant metastasis. Consequently, patients with pancreatic NENs and preoperative glucose abnormalities require vigilant glucose monitoring and stringent glycemic control [54].
Diabetes and NENs
The majority of patients diagnosed with NENs are typically between the ages of 50 and 70 [55], and patients often have complications such as hypertension and diabetes. Numerous studies have identified diabetes as a significant risk factor for the development of NENs [56, 57], systematic reviews and meta-analyses have consistently shown that diabetes is a significant risk factor for the development of pancreatic and gastric neuroendocrine tumors (NETs). Furthermore, the overall risk was higher in insulin-treated diabetic patients, whereas no such association was shown in patients with NETs of the small intestine, rectum, and lung [58, 59]. Capurso et al. demonstrated that a history of diabetes correlates with the presence of distant metastases at the time of diagnosis [57]. The results of another study showed that type 2 diabetes mellitus was associated with pancreatic NET metastasis, but was not an independent risk factor for poor prognosis of pNETs [60].
In contrast, a study by Maria et al., which included 1,535 patients with NETs who underwent somatostatin analog therapy, revealed that comorbid diabetes did not contribute to increased mortality among these patients [61]. Kusne et al. conducted a study matching newly diagnosed NENs with or without diabetes by age, gender and year of diagnosis in a 1:1 ratio, and compared survival rates and blood glucose and glycosylated hemoglobin levels within one year post-diagnosis. The results indicated that diabetes did not exert an adverse effect on the survival of NENs patients, and the presence of NETs and their treatment did not impact glycemic control [62].
NENs are also often associated with the development of diabetes or impaired glucose tolerance, particularly in patients with pancreatic NENs. Functional NENs, such as glucagonomas and somatostatinomas, can trigger hyperglycemia and insulin resistance by secreting hormones like glucagon and somatostatin. Additionally, in patients with pancreatic NENs who undergo surgical treatment, glucose metabolism may be further compromised due to reduced insulin secretion [63]. From this perspective, diabetes might represent an early paraneoplastic state or the result of tumor-induced disruptions in glucose metabolism, rather than an instigator of tumorigenesis [64].
At present, the causal relationship and mechanism between dysglycemia and the development of NENs remains insufficiently explored, with existing literature often presenting conflicting findings. And most of the studies do not provide data on potentially corrective factors, such as diet, nutrition, physical activity, and type of diabetic treatment. Therefore, there is a clear need for further well-designed prospective studies to elucidate this complex relationship.
Metformin and NENs
Metformin, a widely prescribed hypoglycemic agent for diabetes management, has been shown in numerous studies to possess anticancer properties, potentially improving the prognosis of cancer patients. However, research on the specific impact of metformin on the progression and prognosis of NENs is comparatively scarce [65]. A retrospective study of 445 patients with advanced pancreatic NENs revealed an unexpected finding: patients with comorbid diabetes had significantly longer progression-free survival (PFS) than those without diabetes. Further analysis indicated that among diabetic patients, those treated with metformin exhibited even more pronounced PFS benefits over non-diabetic patients and those receiving alternative treatments. Notably, multivariate analyses, which adjusted for various outcome-associated factors, demonstrated that metformin was associated with longer PFS, whereas blood glucose levels were not [66].
Pusceddu et al. investigated the potential correlation between metformin treatment and the prognosis of patients with pancreatic NENs who were concurrently receiving everolimus and octreotide [67]. To elucidate the anti-tumor effects of metformin in NENs, several clinical trials are underway. A phase 2 trial at the Cancer Research Institute of São Paulo, Brazil (NCT02279758), is assessing the therapeutic efficacy of metformin in patients with gastroenteropancreatic NENs (GEP-NENs). Concurrently, a prospective phase II trial exploring the efficacy of metformin plus everolimus and octreotide (NCT02294006) and a phase I trial on the safety of metformin plus lanreotide in the treatment of gastrointestinal and pulmonary NENs (NCT02823691) are under way in Italy.
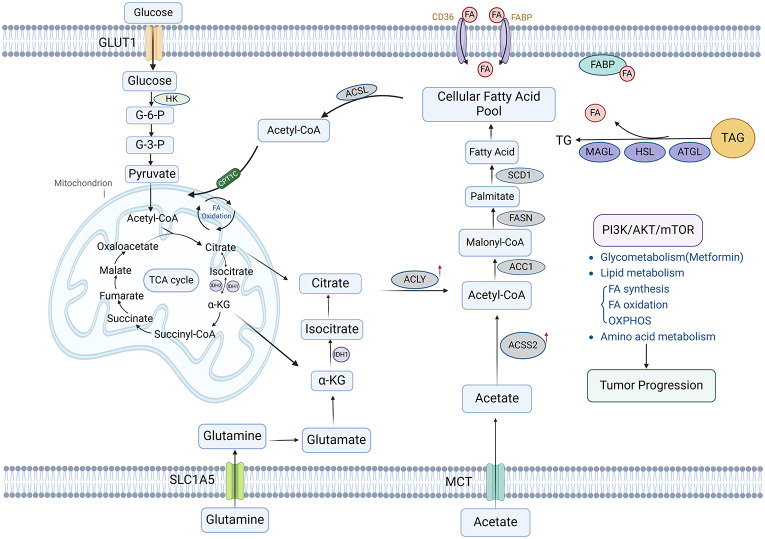
Numerous preclinical studies have investigated the impact of metformin on NEN -derived cell lines, as well as the underlying mechanisms. These studies have demonstrated that metformin can suppress the proliferation and migration of the NEN-derived cell lines, QGP-1 and BON-1, in a time-dependent fashion [68]. Further research has revealed that with the increase of metformin concentration, the viability of NEN-derived cell lines were inhibited in a dose-dependent manner. This effect was attributed to metformin’s ability to suppress the mTOR signaling pathway in the cells, in which glycogen synthase kinase 3 (GSK3) played a pivotal role [69]. Yamana et al. demonstrated that metformin could inhibit the proliferation and tumor growth of pancreatic NEN cell lines QGP-1 by inducing cell cycle arrest and apoptosis. Additionally, metformin has been shown to down-regulate the expression of the angiogenesis-associated protein TIMP-1 and its corresponding microRNA in QGP-1 cells [70]. There is still a lack of in-depth research about the effects of metformin on the proliferation, invasion and other capabilities of NENs and the specific molecular mechanisms (Fig. 1, Fig. S1).
Fig. 1.
Schematic overview of metabolic pathways in NENs. PI3K/AKT/mTOR pathway plays an important role in the glycometabolism, lipid, and amino acid metabolism processes of NENs
Lipid metabolism changes and NENs
Dyslipidaemia and NENs
Lipid metabolism disorder is an important risk factor for the progression of NENs, and regulating lipid metabolism might be a potential therapeutic target [71]. A prospective diet and health study, which included half a million participants, identified 60 cases of adenocarcinoma and 80 cases of carcinoid tumors of the small bowel over an 8-year follow-up period. The analysis revealed that a high intake of saturated fats was significantly associated with an increased risk of small bowel NENs [72]. A retrospective clinical study on pheochromocytomas and paragangliomas (PPGLs) reported a 46% prevalence of concomitant hyperlipidemia among PPGL patients, and hyperlipidemia could be improved to some extent by surgical resection [73].
In a retrospective case-control study encompassing 102 patients with histologically confirmed rectal NETs undergoing screening colonoscopy, and 52,583 healthy controls, logistic regression analysis revealed that elevated cholesterol levels were significantly associated with the risk of developing rectal NETs [74]. An analysis of clinical data from 146 patients with nonfunctioning pituitary neuroendocrine tumors (NF-PitNETs) demonstrated that high cholesterol level was an independent risk factor for the development of cavernous sinus invasion in these patients [75].
Fatty acid metabolism and NENs
Acetyl coenzyme A (acetyl-CoA) serves as the sole carbon source and precursor for fatty acid biosynthesis in mammalian cells. It is produced from citrate by the action of ATP-citrate lyase (ACLY) and from acetate by the action of acetyl-CoA synthetase (ACSS). Dysregulation of the fatty acid synthesis has significant implications for tumor development [76]. ACLY has been identified with elevated expression levels in melanoma and is known to specifically activate the MITF-PGC1α axis, thereby driving cell proliferation and enhancing tumor growth [77]. Our previous study found that the expression of acetyl-CoA synthetase (ACSS2) increased in pNENs under hypoxia, which facilitated the progression of pNENs by modulating lipid metabolism changes through the PI3K/AKT/mTOR pathway [78].
Fatty acid synthase (FASN) is another crucial enzyme in the fatty acid synthesis process. Previous research has demonstrated that FASN was highly expressed in NENs and played a pivotal role in tumor development, as confirmed by both in vitro and in vivo experiments. Moreover, we found that orlistat, an inhibitor of FASN, could counteract its pro-tumorigenic effects in NENs [79]. Fatty acid binding proteins (FABPs) serve as essential lipid transporters, facilitating the delivery of lipids to specific cellular compartments, sequestering lipid droplets, mediating intra- and extra-cellular signaling, aiding in membrane synthesis, and modulating the activity of other enzymes and lipid-regulated transcription factors. Our previous study has identified elevated FABP5 expression in pNENs tissues and cells. Subsequent mechanistic investigations have uncovered that FABP5 modulated FASN expression via the ubiquitin-proteasome pathway and activated the Wnt/β-catenin signaling pathway to promote the progression of pNENs [79]. Additionally, FABP5 expression could be up-regulated by m6A demethylase ALKBH5 in an m6A-IGF2BP2 dependent manner, which activated PI3K/Akt/mTOR signaling pathway, leading to lipid metabolism disorders and promoting the progression of pNENs [80].
Stearoyl-CoA desaturase (SCD) is an iron-dependent enzyme that catalyzes the rate-limiting step of unsaturated fatty acid synthesis. A growing body of research indicates that SCD1 is a pivotal regulator of ferroptosis, a form of cell death dependent on iron. Targeted metabolomics analysis has revealed that overexpression of the MEN1 gene enhances the production and oxidation of polyunsaturated fatty acids in QGP-1 cells. Lipid peroxidation, a characteristic of ferroptosis, has been further implicated by studies showing that MEN1 regulates SCD1-mediated ferroptosis by inhibiting the mTOR signaling pathway. Notably, oleic acid, a metabolite of SCD1, has been found to alleviate the lipid peroxidation induced by MEN1 overexpression [81].
Cholesterol metabolism and NENs
Oxysteroids, products of cholesterol oxidation, have been shown to promote tumor growth both directly by enhancing tumor cell proliferation and indirectly by suppressing antitumor immune responses. Studies have found that hypoxia-inducible factor-1α (HIF-1α) regulated the overexpression of the Cyp46a1 enzyme in pNENs, responsible for generating the oxysterol 24-hydroxycholesterol (24 S-HC). The activation of the HIF-1α/24S-HC axis has been shown to directly induce neoangiogenesis by targeting pro-angiogenic neutrophils to hypoxic areas that necessitate neovascularization [82].
Bioinformatics analysis of the GSE169498 dataset, contrasting immune cell infiltration in invasive versus non-invasive non-functional pituitary neuroendocrine tumors (NF-PitNETs), has uncovered a potential role for cholesterol metabolism-related genes. These genes may enhance immune cell infiltration in NF-PitNETs and facilitate the tumors’ invasion of the cavernous sinus via mTOR signaling. This finding offers a novel perspective for investigating the pathogenesis of cavernous sinus invasion in NF-PitNETs and for identifying potential therapeutic targets for this condition [75] (Fig. 2).
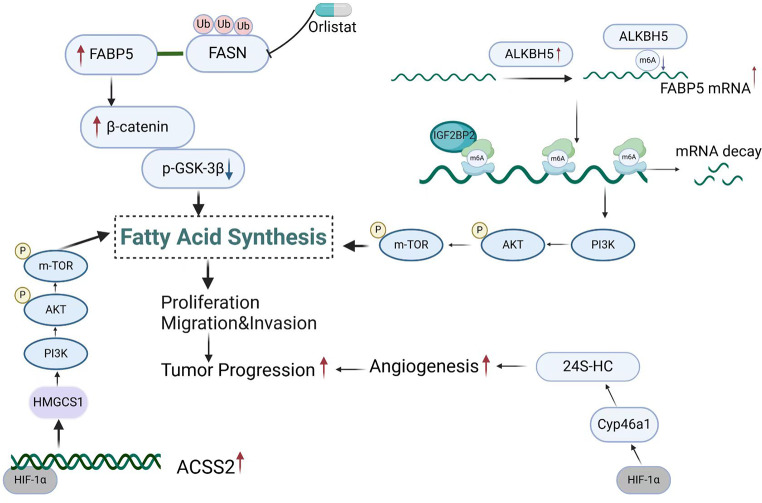
Fig. 2.
Lipid metabolism changes in NENs. The expression of ACSS2 is up-regulated by HIF-1α under hypoxia, which regulates lipid metabolism through the PI3K/AKT/mTOR pathway to promote the progression of NENs. FASN, a key enzyme in the fatty acid synthesis pathway, is up-regulated in NEN. FABP5 interacts with FASN to regulate the expression of FASN through the ubiquitin-proteasome pathway, promote lipid droplet deposition and activate Wnt/β-catenin signaling pathway to promote the progression of NEN. However, the FASN inhibitor orlistat could reverse this process. ALKBH5, an m6A demethylase, is up-regulated in NENs, which increases the expression of FABP5 in an m6A-IGF2BP2-dependent manner, activates PI3K/Akt/mTOR signaling pathway, and leads to lipid metabolism disorders and promotes the progression of NENs. HIF-1α regulates the overexpression of Cyp46a1 enzyme in NENs to produce 24 S-HC, and activation of the HIF-1α/ 24 S-HC axis induces neovascularization
Metabolic syndrome and NENs
Obesity, characterized by a body mass index (BMI) of 30 or higher, is a component of a cluster of metabolic disorders collectively known as metabolic syndrome (MS). MS is diagnosed when three or more of the following conditions are present: (1) central obesity, defined as a waist circumference of 102 cm or more in males and 85 cm or more in females; (2) hypertriglyceridemia, indicated by fasting triglyceride levels of 1.7 mmol/L or higher; (3) reduced fasting high-density lipoprotein cholesterol (HDL-C) levels, with thresholds of less than 1.0 mmol/L for males and less than 1.3 mmol/L for females; (4) hypertension, identified by blood pressure readings of 135/85 mmHg or higher, or the use of anti-hypertensive medication; (5) impaired fasting glucose, with levels of 6.1 mmol/L or higher, or glucose levels of 7.8 mmol/L or higher at 2 h after glucose loading, and/or diabetes mellitus has been diagnosed and treated [83]. Numerous studies have demonstrated that metabolic syndrome is a significant risk factor for NENs.
Obesity and NENs
Research indicates that obesity is an independent risk factor for the development of NENs. A significant number of patients with gastrointestinal NENs were identified incidentally during routine endoscopic evaluations conducted prior to bariatric surgery. The estimated incidence of gastric NETs in obese individuals was 0.23 to 0.358%, while it was 0.001 to 0.002% in the general population [84]. Do weight-loss interventions affect outcomes in patients with diagnosed NENs? A systematic review suggested that weight loss might confer some benefit on cancer-related mortality [85].
However, contradictory evidence suggested that a higher BMI might confer a potential protective effect on the outcomes of GEP-NENs [86]. Specifically, research by Hassan et al. demonstrated a 60–70% reduction in the risk of pancreatic and small intestinal NETs in overweight and obese individuals [59]. Carcinoid syndrome and treatments for NENs could result in poor nutritional status and weight loss, with malnutrition affecting up to 25% of patients with GEP-NENs. This condition was linked to a nearly fivefold increase in mortality rates and has a negative impact on the prognosis of patients [87]. Currently, there are no specific guidelines for the management of obese patients diagnosed with GEP-NENs, and there is a dearth of prospective clinical trials in this area. This underscores the need for a comprehensive and individualized approach to care that addresses the unique nutritional requirements of each patient, rather than focusing solely on achieving specific BMI targets.
Metabolic syndrome and NENs
In a case-control study, 96 patients with well-differentiated GEP-NETs were cross-matched with controls from the general population (n = 96) for age, gender, and region of residence. Further analysis revealed that well-differentiated GEP-NETs were significantly associated with MS criteria and its individual components, including visceral obesity, fasting triglycerides, and fasting blood glucose levels. The correlation intensified when individuals exhibited four or more metabolic disorders characteristic of MS [88]. Subsequent follow-up studies within the same cohort revealed that patients with metastatic GEP-NENs exhibited a significantly elevated risk of MS. Moreover, those with comorbid GEP-NENs and MS presented with higher tumor grading and an increased risk of metastasis. This positive correlation between MS and the characteristics of GEP-NENs underscores the potential interplay between these two conditions, warranting further investigation into their shared pathophysiological mechanisms [89].
The fatty liver index (FLI) serves as a non-invasive diagnostic tool for identifying individuals with non-alcoholic fatty liver disease, while the visceral adiposity index (VAI) is recognized as a gender-specific indicator of lipid dysfunction. Both indices are regarded as early predictors of MS. In a study that included 109 pathologically confirmed G1/G2 phase GEP-NETs patients and 109 age-, gender-, and BMI-matched healthy controls, the findings indicated that the VAI and FLI values, as well as the prevalence of MS, were significantly higher in the GEP-NET patient group compared to the healthy controls. Notably, these indices and the incidence of MS were more pronounced in G2 grade patients than in G1 grade patients. Furthermore, patients with advanced or metastatic GEP-NET exhibited higher VAI and FLI values and a greater incidence of MS than those without metastasis. Importantly, elevated VAI and FLI values, along with a higher prevalence of MS, were correlated with a poorer clinical prognosis in GEP-NET patients [90].
Amino acid metabolism changes and NENs
Smith et al. conducted research utilizing genetically engineered glucagon receptor (GCGR) knockout mice or GCGR inhibitory antibodies to investigate the cellular origins and signaling pathways that initiate and promote the progression of mice pNETs. Their study demonstrated that elevated plasma amino acid levels stimulated the proliferation of SLC38A5-positive embryonic progenitor-like alpha cell populations in mice. Additionally, significant expression of SLC38A5 homolog SLC7A8 was found in tumor cells from pNEN patients, as well as activated mTOR signaling markers. Based on these findings, it could inferred that human alpha cells might generate progenitor cells in response to metabolic signals, and that these progenitor cells could drive tumor initiation when exposed to persistent inducing signals [91]
A hallmark of functional NENs with carcinoid syndrome is the substantial production and release of serotonin. This neurotransmitter is synthesized through a two-step process: the conversion of tryptophan (Trp) to 5-hydroxytryptamine (5-HTP) by tryptophan hydroxylase 1 (TPH1), followed by the decarboxylation of 5-HTP to serotonin by aromatic amino acid decarboxylase (AAAD). Typically, only about 1% of dietary tryptophan is converted to 5-hydroxytrypatmine (5-HT) in individuals without carcinoid syndrome. In stark contrast, in patients with carcinoid syndrome, an elevated proportion-up to 60%-of dietary tryptophan is converted into serotonin within tumors, leading to the clinical manifestations of the syndrome [92]. Gilbert et al. discovered that the maximum velocity (Vmax) of AAAD in NEN tumor tissue was over 50 times greater than that observed in normal liver tissue. Western blotting analysis revealed that the AAAD polypeptide content in NEN tissue exceeded that of the adjacent normal liver tissue by more than 20-fold. These findings suggested that AAAD could represent a promising target for the development of enzyme-activated prodrug therapies for the treatment of NENs [93].
To evaluate the potential of targeting elevated AAAD levels for chemotherapy, Gilbert et al. conducted an experiment where NCI-H727 human lung neuroendocrine tumor cells were incubated for 72 h with three distinct AAAD inhibitors: carbidopa, 3-hydroxybenzylhydrazine (NSD-1015), and alpha-monofluoromethyldopa (MFMD). The results indicated that both carbidopa and MFMD exhibited lethal effects, whereas NSD-1015 showed no impact on cell proliferation. Further analysis revealed that carbidopa’s lethality was selective, only affecting the NCI-H146 and NCI-H209 small cell lung cancer (SCLC) cell lines, without influencing the proliferation of DU145 prostate cancer, MCF7 breast cancer, and NCI-H460 large cell lung cancer cell lines. Among lung tumor cell lines, which including carcinoid, two SCLC cell lines, and one large cell lung cancer line, the cytotoxicity induced by carbidopa was found to in correlation with AAAD activity levels. This selectivity suggests that carbidopa, an AAAD inhibitor, possesses specific cytotoxic properties against human lung carcinoid and SCLC cells [94].
In addition to AAAD, TPH1 is also a pivotal enzyme in the synthesis of serotonin (5-HT). Telotristat, a novel TPH inhibitor, has been approved for clinical use in patients with carcinoid syndromes who experience uncontrolled diarrhea despite somatostatin analog therapy. In vitro studies with human pNET cells demonstrated that Telotristat significantly reduced 5-HT production in a dose-dependent manner within clinically relevant concentrations, without affecting cell proliferation. When combined with pasireotide, but not octreotide, Telotristat exhibited additive inhibitory effects on 5-HT secretion. Furthermore, 5-HT was found to lack autocrine effects on NET cell proliferation in a 3D cell model. These findings indicate that Telotristat is an effective inhibitor of 5-HT synthesis. However, the efficacy of combining Telotristat with somatostatin receptor 2-biased analogs warrants further detailed evaluation [94] (Fig. 3).
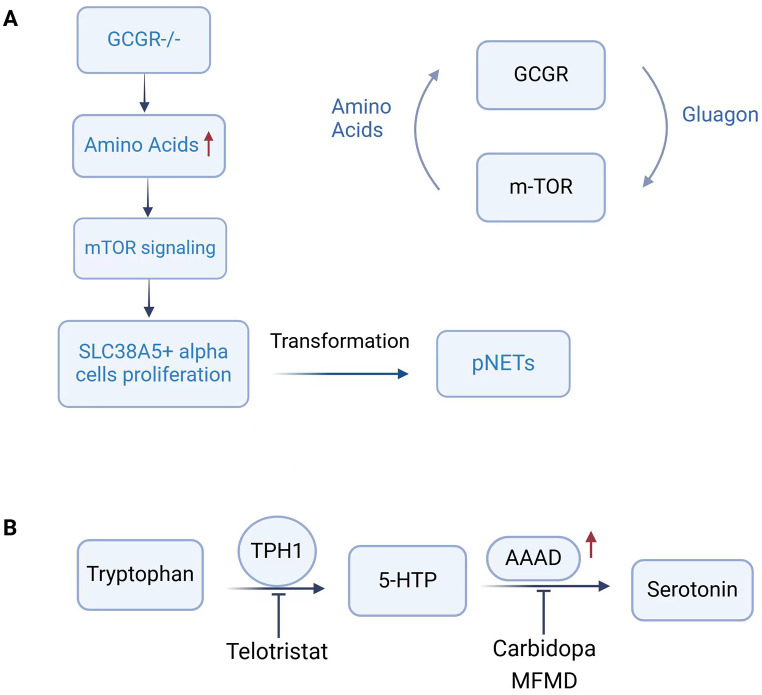
Fig. 3.
Schematic diagram of amino acid metabolism in NENs. (A) GCGR knockout mice exhibit elevated plasma amino acids and activation of mTOR signaling, driving the proliferation of mouse SLC38A5 + embryonic progenior-like α cell population, which drives tumor initiation when the inducing signal is prolonged. (B) TPH1 converts tryptophan to 5-HTP, which is then decarboxylated by AAAD to synthesize serotonin. Carbidopa and MFMD, inhibitors of AAAD, and Telotristat, a novel inhibitor of TPH, inhibit serotonin synthesis and ameliorate the carcinoid syndrome
Metabolomics study of NENs
Metabolomics offers an expansive platform for disease monitoring and therapeutic efficacy assessment by enabling high-throughput analysis of all small molecule metabolites present in biological samples, such as blood, urine, and tissues. This approach involves the screening of endogenous small molecule compounds, followed by an in-depth analysis of differential metabolic pathways. Ultimately, it aids in the identification of representative biomarkers that can signify the progression of diseases or the effectiveness of treatments [95]. Metabolomic data hold a significant advantage over genomic or proteomic approaches due to their comprehensive inclusion of gene-environment interactions. Factors such as drug effects, dietary influences, and microbiome activity, which are recognized for their substantial impact on tumorigenesis, disease progression, and the efficacy and toxicity of cancer therapies, are all captured within this realm [96].
A comprehensive metabolomic study, encompassing 94 small intestine NETs (SI-NETs), 18 liver metastases from NETs, and 30 normal small intestine (SI) and liver tissue samples, identified and quantified 27 key metabolites. This analysis aimed to assess the metabolic differences between primary SI-NETs and normal SI tissues, as well as between primary SI-NETs and NETs with liver metastases. The findings revealed significant increases in the levels of succinate, glutathione, taurine, inositol, and glycerophosphocholine in SI-NETs, in contrast to normal SI samples, which exhibited higher levels of alanine, creatine, ethanolamine, and aspartate. Notably, among the more aggressive SI-NETs, concentrations of glucose, serine, and glycine were found to be lower, whereas levels of choline-containing compounds, taurine, lactic acid, and alanine were elevated. In the context of metastatic liver NET, the study observed a significant elevation in the levels of acetate, succinate, choline, phosphocholine, taurine, lactic acid, and aspartic acid compared to normal liver tissues. Additionally, liver metastases displayed significantly higher levels of alanine, ethanolamine, glycerophosphocholine, and glucose than those found in primary SI-NETs tissues [97].
In a single-center, prospective, controlled observational study involving 34 untreated patients with NENs, metabolomic analysis of urine samples revealed significant alterations in tryptophan metabolism. Specifically, secondary metabolites of tryptophan, including trigonelline and a metabolite associated with nicotinic acid, were found to be generally reduced in NENs patients. In contrast, upstream metabolites like kynurenine exhibited increased levels. Additionally, hippuric acid, a metabolite derived from the gut, was observed to be decreased across all NEN patients. And other gut microbial co-metabolites, such as trimethylamine-N-oxide, 4-hydroxyphenylacetate, and phenylacetylglutamine showed elevated levels in SI-NETs. These findings suggest an intricate interplay between the regulatory systems of tumor metabolism, neuroendocrine signaling molecules, and gut microbial co-metabolism in the pathophysiology of NENs [98]. In a prospective study involving 28 patients diagnosed with NENs, metabolomic analysis of urine samples indicated that different NENs subgroups exhibit distinct metabolic profiles. These findings suggest the potential for stratified metabolic phenotypes within the NENs patients, highlighting the importance of personalized approaches in disease management [99].
An untargeted metabolomics analysis utilizing plasma samples from 77 patients with NETs and 68 healthy controls identified 155 distinct compounds. This study revealed an increase in bile acids, sugars, oxidized lipids, and arachidonic acid oxidation products in the plasma of NETs patients, along with a decrease in carnitine levels. Additionally, 32 metabolic pathways were found to be significantly enriched in NETs, particularly those associated with the tricarboxylic acid (TCA) cycle and amino acid metabolism. Ultimately, orthogonal partial least squares-discriminant analysis (OPLS-DA) and receiver operating characteristic (ROC) analysis were employed to screen 48 metabolites that demonstrated diagnostic potential [100]. In a multi-platform, non-targeted metabolomic analysis of plasma samples from 77 patients with G1-2 extrapancreatic NETs enrolled in the AXINET trial (NCT01744249), and 68 non-cancer controls, 34 metabolites were identified with significant associations with PFS and/or overall survival (OS) in NETs patients. Multivariate analysis further revealed that 13 of these metabolites were significant independent prognostic factors, with 3 exerting substantial impacts on both PFS and OS. Upon further examination through Metabolic Set Enrichment Analysis (MSEA) and Metabolic Pathway Analysis (MPA) of the 13 metabolite profiles, it has discovered that methionine, porphyrin, and tryptophan metabolism pathways were the most dysregulated and closely associated with the prognosis of NETs. These findings might pave the way for new potential targets in the development of innovative therapeutic strategies for patients with NETs [101].
Jannin et al. conducted an in-depth analysis of metabolic pathways in pNETs by examining the available transcriptome and metabolome data from the literature. Their findings indicated that several key metabolic processes, including single-carbon transfer, glutathione and polyamine metabolism, fatty acid biosynthesis, and the catabolism of branched-chain amino acids supplying the TCA cycle, were dysregulated in pNETs. This metabolic dysregulation was associated with the proliferation and metastasis of pNET cells and might also hold prognostic significance [102]. In our previous study, we conducted a serum metabolomic analysis comparing metastatic and non-metastatic pNENs, which led to the identification of serum methylmalonic acid (MMA) as an onco-metabolite. This finding was subsequently verified through both in vitro and in vivo experiments. Further mechanistic studies revealed that MMA upregulated the neuroendocrine-specific transcription factor FOXA2, initiating transcriptional activation of INHBA and promoting the progression of pNENs via the Activin A-SMAD2/3-MITF signaling axis. These insights offer a promising new target for the diagnosis and treatment of pNENs [103]. Another non-targeted analysis utilizing plasma samples from a large cancer cohort reported the upregulation of hydroxyeicosatetraenoic acids (HETEs) and oxidized lyso-phosphatidylcholines (oxLPCs) in patients with NENs, revealing the role of lipid oxidation in the metabolic profile of NENs [104]. Although metabonomics has identified numerous potential targets associated with metabolic dysregulation pathways in NENs, additional research is essential to validate the findings from these analyses. This validation process is crucial for the exploration of these targets as potential biomarkers or for the development of novel therapeutic strategies (Fig. 4).
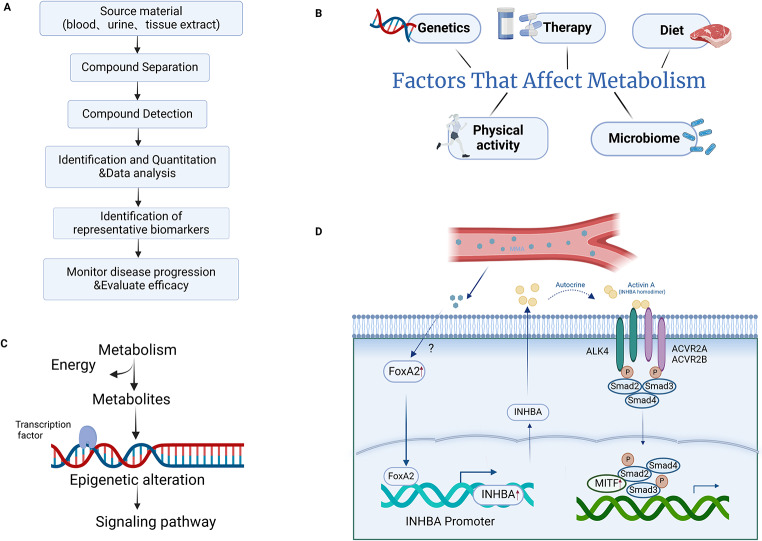
Fig. 4.
Metabolomics study in NENs. (A) The flow chart of metabolomics is shown in the upper left panel. (B) NENs cause metabolic alterations and environmental factors can in turn affect the progression of NENs by affecting metabolism. (C) In addition to providing energy for tumor cells, metabolism can also cause epigenetic changes through metabolites and affect signaling pathways, thereby regulating tumor progression. (D) MMA, a serum metabolite, up-regulates the expression of INHBA through transcription factor FOXA2, which affects the downstream signaling pathways and regulates the progression of NENs
Conclusion & prospects
NENs are a group of highly heterogeneous tumors that originate from neuroendocrine cells widely distributed throughout the body and have a characteristic neuroendocrine phenotype. NENs exhibit a spectrum of biological behaviors ranging from relatively indolent to highly aggressive. Tumors of different origins and whether functional or non-functional, not only present varied clinical features but also display distinct metabolic processes. Metabolic change is essential for the continuous growth and progression of tumors and is recognized as a significant hallmark of tumor progression. Metabolic alteration could facilitate tumor metastasis by modulating specific genes or molecular pathways, with tumor cell metabolic pathways often undergoing alterations during the metastatic process. While a large number of studies have reported the role and mechanisms of metabolic changes in various cancers, the relationship between metabolic changes and the development of NENs, as well as its underlying mechanisms, remains understudied and fragmented. This paper offers a systematic review of the literature exploring the relationship between NENs and metabolic alterations, aiming to provide new perspectives for the study of the mechanisms and therapeutic targets. However, the limitation is that NETs and NECs were not strictly distinguished in this review since most original research on the metabolism of NENs did not clearly distinguish them. In the future, it is necessary to summarize research progress on the metabolism of NETs and NECs separately based on more original studies.
Current research on the relationship between abnormal blood glucose levels and the development of NENs has yielded inconsistent findings. This disparity might stem from the fact that most studies have not accounted for variables such as diet, nutrition, physical activity, and diabetes treatment methods. As a result, the causality between aberrant glucose metabolism and NENs development remains undetermined, necessitating further well-designed prospective studies. Metformin, a widely used hypoglycemic agent, has shown promise in inhibiting the proliferation and migration of NEN cell lines. However, the underlying molecular mechanisms are not yet fully understood and warrant further investigation.
Dyslipidemia has been also identified as a risk factor for NENs development, and surgical removal of NENs might improve hyperlipidemia [73, 74]. Further studies are needed to confirm whether lipid-lowering agents can directly inhibit NENs [105]. Additionally, various enzymes implicated in fatty acid synthesis, lipid transport, and lipid peroxidation have been associated with the progression of NENs. Cholesterol oxidation products, such as oxysterols, and genes related to cholesterol metabolism are suspected to contribute to angiogenesis as well as invasion and metastasis of NENs. While the association between lipid metabolism and NENs development is evident, more in-depth exploration of the underlying molecular mechanisms is still required.
The relationship between obesity and NENs is complex and inconsistent across studies. Some research suggested that obesity might elevate the risk of NENs development, while also indicating that bariatric surgery could potentially improve NENs prognosis. Conversely, other findings suggested that obesity might actually decrease NENs incidence, acting as a protective factor for patient outcomes. Notably, malnourished NENs patients exhibited a higher mortality risk, underscoring the need for personalized nutrition programs and weight management strategies. The incidence of MS was significantly higher in NENs patients, particularly among those with metastatic disease. Furthermore, NENs patients with MS appeared to have an increased likelihood of metastasis. This highlights the importance of focusing on the prevention and management of MS in NENs patients and the necessity for screening NENs in individuals with MS.
Amino acid metabolism is predominantly linked to carcinoid syndrome in NENs patients. Current research on enzyme inhibitors presents promising therapeutic targets for controlling symptoms such as diarrhea associated with carcinoid syndrome. Metabolomics research in NENs offers novel insights for diagnostic and therapeutic approaches. However, given the high heterogeneity of NENs and the variability in metabolic disorders, there is a need for larger-scale metabolomics studies. The findings from these studies require verification and further in-depth exploration of molecular mechanisms to identify new diagnostic and therapeutic targets for NENs.
Various models have recently been developed to study the biology of NENs and identify novel therapeutic treatments, such as organoids, genetically engineered mouse (GEMM) model, and patient-derived xenograft (PDX) models [106–108]. Suitable and reliable preclinical models provide a very valuable basis for the exploration of molecular studies, targeted studies, metabolism-related investigations, and novel therapeutic treatments of NENs. A total of 25 NEN organoids were established and comprehensively characterized. Transcriptome analysis identified molecular subtypes distinguished by the expression of different transcription factors, linking the genetics and biological phenotypes of NENs [106]. Through a comprehensive analysis of more than 1000 NECs from 31 various tissues, Wang et al. revealed molecular differentiation driven by different transcriptional regulators and proposed a unified classification framework that divides NECs into five intrinsic subtypes. Notably, the newly discovered H subtype, dominated by HNF4A, is highly resistant to a series of chemotherapy drugs. Further analysis showed that metabolic reprogramming triggered by the downstream metabolic genes of KEAP1-NRF2 signaling pathway may contribute to its significant resistance to a series of chemotherapy drugs, which is expected to become novel therapeutic targets [109].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization: CHH, QYT, MJY Visualization: CHH, LYC, YD Funding acquisition: MJY Project administration: QYT Supervision: QYT, MJY Writing – original draft: CHH, LYC, YD Writing – review & editing: CHH, QYT, MJY.
Funding
This work was supported by China postdoctoral Science Foundation project (No. 2023M731414), and the National Natural Science Foundation of China (No. 82373180 and 82303453).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing interests.
Footnotes
One sentence summary: We reviewed the relationship between metabolic changes and neuroendocrine neoplasms, and discussed the potential therapeutic strategies.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chunhua Hu, Lingyi Chen and Yi Ding contributed equally to this work.
Contributor Information
Mujie Ye, Email: mujiey0629@163.com.
Qiyun Tang, Email: qytang@njmu.edu.cn.
References
- 1.Rindi G et al (2018) A common classification framework for neuroendocrine neoplasms: an international agency for research on cancer (IARC) and world health organization (WHO) expert consensus proposal. Mod Pathol 31(12):1770–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cives M, Strosberg JR (2018) Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin 68(6):471–487 [DOI] [PubMed] [Google Scholar]
- 3.Lee MR et al (2019) Incidence trends of gastroenteropancreatic neuroendocrine tumors in the united States. Clin Gastroenterol Hepatol 17(11):2212–2217e1 [DOI] [PubMed] [Google Scholar]
- 4.Lamberti G et al (2024) Gastric neuroendocrine neoplasms. Nat Rev Dis Primers 10(1):25 [DOI] [PubMed] [Google Scholar]
- 5.Chauhan A et al (2024) Critical updates in neuroendocrine tumors: version 9 American joint committee on cancer staging system for gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin 74(4):359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallet J et al (2015) Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 121(4):589–597 [DOI] [PubMed] [Google Scholar]
- 7.Hu P et al (2020) Trends of incidence and prognosis of gastric neuroendocrine neoplasms: a study based on SEER and our multicenter research. Gastric Cancer 23(4):591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyld D et al (2019) Epidemiological trends of neuroendocrine tumours over three decades in Queensland, Australia. Cancer Epidemiol 63:101598 [DOI] [PubMed] [Google Scholar]
- 9.Dasari A et al (2017) Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the united States. JAMA Oncol 3(10):1335–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacalle-Gonzalez C et al (2023) Management of non-hepatic distant metastases in neuroendocrine neoplasms. Best Pract Res Clin Endocrinol Metab 37(5):101784 [DOI] [PubMed] [Google Scholar]
- 11.Modlin IM et al (2011) The archaic distinction between functioning and nonfunctioning neuroendocrine neoplasms is no longer clinically relevant. Langenbecks Arch Surg 396(8):1145–1156 [DOI] [PubMed] [Google Scholar]
- 12.Pelosof LC et al (2010) Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc 85(9):838–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guilmette J et al (2019) Paraneoplastic syndromes and other systemic disorders associated with neuroendocrine neoplasms. Semin Diagn Pathol 36(4):229–239 [DOI] [PubMed] [Google Scholar]
- 14.JE I (2006) Current concepts in neuroendocrine cancer metabolism. Pituitary 9(3):193–202 [DOI] [PubMed] [Google Scholar]
- 15.Moertel CG et al (1983) Treatment of the carcinoid tumor and the malignant carcinoid syndrome. J Clin Oncol 1(11):727–740 [DOI] [PubMed] [Google Scholar]
- 16.Ishizuka J et al (1992) Receptor-mediated autocrine growth-stimulatory effect of 5-hydroxytryptamine on cultured human pancreatic carcinoid cells. J Cell Physiol 150(1):1–7 [DOI] [PubMed] [Google Scholar]
- 17.de la Dornonville C et al (2005) Ghrelin treatment reverses the reduction in weight gain and body fat in gastrectomised mice. Gut 54(7):907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagtegaal ID et al (2020) The 2019 WHO classification of tumours of the digestive system. Histopathology 76(2):182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Rosa S, Uccella S (2021) Classification of neuroendocrine neoplasms: lights and shadows. Rev Endocr Metab Disord 22(3):527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uccella S et al (2024) Molecular classification of Gastrointestinal and pancreatic neuroendocrine neoplasms: are we ready for that?? Endocr Pathol 35(2):91–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M et al (2023) The mutational, prognostic, and therapeutic landscape of neuroendocrine neoplasms. Oncologist 28(9):e723–e736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puccini A et al (2020) Comprehensive genomic profiling of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs). Clin Cancer Res 26(22):5943–5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umetsu SE et al (2023) Integrated genomic and clinicopathologic approach distinguishes pancreatic grade 3 neuroendocrine tumor from neuroendocrine carcinoma and identifies a subset with molecular overlap. Mod Pathol 36(3):100065 [DOI] [PubMed] [Google Scholar]
- 24.Uccella S et al (2021) Genomics of High-Grade neuroendocrine neoplasms: Well-Differentiated neuroendocrine tumor with High-Grade features (G3 NET) and neuroendocrine carcinomas (NEC) of various anatomic sites. Endocr Pathol 32(1):192–210 [DOI] [PubMed] [Google Scholar]
- 25.Yachida S et al (2022) Comprehensive genomic profiling of neuroendocrine carcinomas of the Gastrointestinal system. Cancer Discov 12(3):692–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venizelos A et al (2021) The molecular characteristics of high-grade gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 29(1):1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H et al (2022) Genomic characterization reveals distinct mutation landscapes and therapeutic implications in neuroendocrine carcinomas of the Gastrointestinal tract. Cancer Commun (Lond) 42(12):1367–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Principe DR et al (2024) Loss of STK11 suppresses lipid metabolism and attenuates KRAS-Induced immunogenicity in patients with Non-Small cell lung cancer. Cancer Res Commun 4(8):2282–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clem BF et al (2012) Molecular pathways: regulation of metabolism by RB. Clin Cancer Res 18(22):6096–6100 [DOI] [PubMed] [Google Scholar]
- 30.Mei W et al (2023) Characteristics of small pancreatic neuroendocrine tumors and risk factors for invasion and metastasis. Front Endocrinol (Lausanne) 14:1140873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang P et al (2021) Clinicopathological features and lymph node and distant metastasis patterns in patients with gastroenteropancreatic mixed neuroendocrine-non-neuroendocrine neoplasm. Cancer Med 10(14):4855–4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Z et al (2019) Incidence and risk factors of Gastrointestinal neuroendocrine neoplasm metastasis in liver, lung, bone, and brain: A population-based study. Cancer Med 8(17):7288–7298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riihimaki M et al (2016) The epidemiology of metastases in neuroendocrine tumors. Int J Cancer 139(12):2679–2686 [DOI] [PubMed] [Google Scholar]
- 34.Mohamed A, Strosberg JR (2019) Medical management of gastroenteropancreatic neuroendocrine tumors: current strategies and future advances. J Nucl Med 60(6):721–727 [DOI] [PubMed] [Google Scholar]
- 35.Kaltsas GA et al (2004) The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev 25(3):458–511 [DOI] [PubMed] [Google Scholar]
- 36.Hanahan D (2022) Hallmarks of cancer: new dimensions. Cancer Discov 12(1):31–46 [DOI] [PubMed] [Google Scholar]
- 37.Faubert B et al (2020) Metabolic reprogramming and cancer progression. Science, 368(6487) [DOI] [PMC free article] [PubMed]
- 38.Sullivan LB et al (2016) Altered metabolite levels in cancer: implications for tumour biology and cancer therapy. Nat Rev Cancer 16(11):680–693 [DOI] [PubMed] [Google Scholar]
- 39.Boroughs LK, DeBerardinis RJ (2015) Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 17(4):351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward PS, Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21(3):297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agnihotri S, Zadeh G (2016) Metabolic reprogramming in glioblastoma: the influence of cancer metabolism on epigenetics and unanswered questions. Neuro Oncol 18(2):160–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Q et al (2023) Metabolic reprogramming in nasopharyngeal carcinoma: mechanisms and therapeutic opportunities. Biochim Biophys Acta Rev Cancer 1878(6):189023 [DOI] [PubMed] [Google Scholar]
- 43.Venneti S, Thompson CB (2017) Metabolic reprogramming in brain tumors. Annu Rev Pathol 12:515–545 [DOI] [PubMed] [Google Scholar]
- 44.Wettersten HI et al (2017) Metabolic reprogramming in clear cell renal cell carcinoma. Nat Rev Nephrol 13(7):410–419 [DOI] [PubMed] [Google Scholar]
- 45.Yu Z et al (2022) Metabolic reprogramming in hematologic malignancies: advances and clinical perspectives. Cancer Res 82(17):2955–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavlova NN, Thompson CB (2016) Emerg Hallm Cancer Metabolism Cell Metab 23(1):27–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pavlova NN et al (2022) The hallmarks of cancer metabolism: still emerging. Cell Metab 34(3):355–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bose S, Le A (2018) Glucose metabolism in cancer. Adv Exp Med Biol 1063:3–12 [DOI] [PubMed] [Google Scholar]
- 49.Li JT et al (2013) [Effects of high glucose on in vitro invasiveness of human breast cancer cell line MDA-MB-435]. Zhonghua Yi Xue Za Zhi 93(2):89–92 [PubMed] [Google Scholar]
- 50.Alisson-Silva F et al (2013) Increase of O-glycosylated oncofetal fibronectin in high glucose-induced epithelial-mesenchymal transition of cultured human epithelial cells. PLoS ONE 8(4):e60471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han L et al (2013) Indometacin ameliorates high glucose-induced proliferation and invasion via modulation of e-cadherin in pancreatic cancer cells. Curr Med Chem 20(33):4142–4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elgendy M et al (2019) Combination of hypoglycemia and Metformin impairs tumor metabolic plasticity and growth by modulating the PP2A-GSK3beta-MCL-1 axis. Cancer Cell 35(5):798–815e5 [DOI] [PubMed] [Google Scholar]
- 53.Zhang P et al (2022) Hyperglycemia is associated with adverse prognosis in patients with pancreatic neuroendocrine neoplasms. Endocrine 77(2):262–271 [DOI] [PubMed] [Google Scholar]
- 54.Sandini M et al (2020) Pre-operative dysglycemia is associated with decreased survival in patients with pancreatic neuroendocrine neoplasms. Surgery 167(3):575–580 [DOI] [PubMed] [Google Scholar]
- 55.Sackstein PE et al (2018) Epidemiologic trends in neuroendocrine tumors: an examination of incidence rates and survival of specific patient subgroups over the past 20 years. Semin Oncol 45(4):249–258 [DOI] [PubMed] [Google Scholar]
- 56.Haugvik SP et al (2015) Diabetes, smoking, alcohol use, and family history of cancer as risk factors for pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Neuroendocrinology 101(2):133–142 [DOI] [PubMed] [Google Scholar]
- 57.Capurso G et al (2009) Risk factors for sporadic pancreatic endocrine tumors: a case-control study of prospectively evaluated patients. Am J Gastroenterol 104(12):3034–3041 [DOI] [PubMed] [Google Scholar]
- 58.Leoncini E et al (2016) Risk factors for neuroendocrine neoplasms: a systematic review and meta-analysis. Ann Oncol 27(1):68–81 [DOI] [PubMed] [Google Scholar]
- 59.Hassan MM et al (2008) Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int J Cancer 123(4):867–873 [DOI] [PubMed] [Google Scholar]
- 60.Fan Z et al (2020) Diabetes is associated with the metastasis of pancreatic neuroendocrine tumors. Pancreas 49(6):751–756 [DOI] [PubMed] [Google Scholar]
- 61.Umlauft M et al (2017) Diabetes mellitus and its effects on All-Cause mortality after radiopeptide therapy for neuroendocrine tumors. J Nucl Med 58(1):97–102 [DOI] [PubMed] [Google Scholar]
- 62.Kusne YN et al (2021) Implications of neuroendocrine tumor and diabetes mellitus on patient outcomes and care: a matched case-control study. Future Sci OA 7(5):FSO684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Resmini E et al (2009) Secondary diabetes associated with principal endocrinopathies: the impact of new treatment modalities. Acta Diabetol 46(2):85–95 [DOI] [PubMed] [Google Scholar]
- 64.Pannala R et al (2008) Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 134(4):981–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thakur S et al (2019) The role of an anti-diabetic drug Metformin in the treatment of endocrine tumors. J Mol Endocrinol 63(2):R17–R35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pusceddu S et al (2018) Metformin use is associated with longer Progression-Free survival of patients with diabetes and pancreatic neuroendocrine tumors receiving everolimus and/or somatostatin analogues. Gastroenterology 155(2):479–489e7 [DOI] [PubMed] [Google Scholar]
- 67.Pusceddu S et al (2016) Metformin with everolimus and octreotide in pancreatic neuroendocrine tumor patients with diabetes. Future Oncol 12(10):1251–1260 [DOI] [PubMed] [Google Scholar]
- 68.Herrera-Martinez AD et al (2019) Type 2 diabetes in neuroendocrine tumors: are Biguanides and Statins part of the solution?? J Clin Endocrinol Metab 104(1):57–73 [DOI] [PubMed] [Google Scholar]
- 69.Vlotides G et al (2014) Anticancer effects of Metformin on neuroendocrine tumor cells in vitro. Horm (Athens) 13(4):498–508 [DOI] [PubMed] [Google Scholar]
- 70.Yamana H et al (2020) Metformin inhibits proliferation and tumor growth of QGP-1 pancreatic neuroendocrine tumor cells by inducing cell cycle arrest and apoptosis. Anticancer Res 40(1):121–132 [DOI] [PubMed] [Google Scholar]
- 71.Modica R (2022) Lipid metabolism and homeostasis in patients with neuroendocrine neoplasms: from risk factor to potential therapeutic target. Metabolites 11(12):1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cross AJ et al (2008) A prospective study of meat and fat intake in relation to small intestinal cancer. Cancer Res 68(22):9274–9279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Good ML et al (2020) Surgical resection of pheochromocytomas and paragangliomas is associated with lower cholesterol levels. World J Surg 44(2):552–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pyo JH et al (2016) Evaluation of the risk factors associated with rectal neuroendocrine tumors: a big data analytic study from a health screening center. J Gastroenterol 51(12):1112–1121 [DOI] [PubMed] [Google Scholar]
- 75.Feng T et al (2023) Identification of cholesterol metabolism-related subtypes in nonfunctioning pituitary neuroendocrine tumors and analysis of immune infiltration. Lipids Health Dis 22(1):127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rohrig F, Schulze A (2016) The multifaceted roles of fatty acid synthesis in cancer. Nat Rev Cancer 16(11):732–749 [DOI] [PubMed] [Google Scholar]
- 77.Guo W et al (2020) ATP-Citrate lyase epigenetically potentiates oxidative phosphorylation to promote melanoma growth and adaptive resistance to MAPK Inhibition. Clin Cancer Res 26(11):2725–2739 [DOI] [PubMed] [Google Scholar]
- 78.Gu D et al (2024) Hypoxia upregulating ACSS2 enhances lipid metabolism reprogramming through HMGCS1 mediated PI3K/AKT/mTOR pathway to promote the progression of pancreatic neuroendocrine neoplasms. J Transl Med 22(1):93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu F et al (2023) FABP5 regulates lipid metabolism to facilitate pancreatic neuroendocrine neoplasms progression via FASN mediated Wnt/beta-catenin pathway. Cancer Sci 114(9):3553–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen J et al (2023) ALKBH5 enhances lipid metabolism reprogramming by increasing stability of FABP5 to promote pancreatic neuroendocrine neoplasms progression in an m6A-IGF2BP2-dependent manner. J Transl Med 1(21):741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye Z et al (2022) MEN1 promotes ferroptosis by inhibiting mTOR-SCD1 axis in pancreatic neuroendocrine tumors. Acta Biochim Biophys Sin (Shanghai) 54(11):1599–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soncini M et al (2016) 24-Hydroxycholesterol participates in pancreatic neuroendocrine tumor development. Proc Natl Acad Sci U S A 113(41):E6219–E6227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eckel RH et al (2005) The metabolic syndrome. Lancet 365(9468):1415–1428 [DOI] [PubMed] [Google Scholar]
- 84.Al-Harbi O et al (2013) Gastric carcinoid and obesity: association or coincidence? Report of two cases and literature review. Case Rep Gastrointest Med 2013:p848075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma C et al (2017) Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ 359:j4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Glazer E et al (2014) Decreased Inpatient Mortality in Obese Patients with Abdominal Nets. Endocr Pract,: pp. 1–20 [DOI] [PubMed]
- 87.Gallo M et al (2019) The management of neuroendocrine tumours: A nutritional viewpoint. Crit Rev Food Sci Nutr 59(7):1046–1057 [DOI] [PubMed] [Google Scholar]
- 88.Santos AP et al (2018) Visceral obesity and metabolic syndrome are associated with Well-Differentiated gastroenteropancreatic neuroendocrine tumors. Cancers (Basel), 10(9). [DOI] [PMC free article] [PubMed]
- 89.Santos AP et al (2019) Disseminated Well-Differentiated Gastro-Entero-Pancreatic tumors are associated with metabolic syndrome. J Clin Med, 8(9) [DOI] [PMC free article] [PubMed]
- 90.Barrea L et al (2021) Cardio-Metabolic indices and metabolic syndrome as predictors of clinical severity of gastroenteropancreatic neuroendocrine tumors. Front Endocrinol (Lausanne) 12:649496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith DK et al (2020) Elevated serum amino acids induce a subpopulation of alpha cells to initiate pancreatic neuroendocrine tumor formation. Cell Rep Med 1(5):100058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shibusawa N, Mori M (2006) [Serotonin producing tumors (carcinoid tumors and carcinoid syndrome)]. Nihon Rinsho, Suppl 3: p. 324–327 [PubMed]
- 93.Gilbert JA et al (1995) Elevated aromatic-L-amino acid decarboxylase in human carcinoid tumors. Biochem Pharmacol 50(6):845–850 [DOI] [PubMed] [Google Scholar]
- 94.Gilbert JA et al (2000) The aromatic-L-amino acid decarboxylase inhibitor carbidopa is selectively cytotoxic to human pulmonary carcinoid and small cell lung carcinoma cells. Clin Cancer Res 6(11):4365–4372 [PubMed] [Google Scholar]
- 95.Schmidt DR et al (2021) Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J Clin 71(4):333–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clayton TA et al (2006) Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature 440(7087):1073–1077 [DOI] [PubMed] [Google Scholar]
- 97.Imperiale A et al (2019) Metabolomics of small intestine neuroendocrine tumors and related hepatic metastases. Metabolites, 9(12) [DOI] [PMC free article] [PubMed]
- 98.Jimenez B et al (2021) Neuroendocrine neoplasms: identification of novel metabolic circuits of potential diagnostic utility. Cancers (Basel), 13(3) [DOI] [PMC free article] [PubMed]
- 99.Kinross JM et al (2013) Metabonomic profiling: a novel approach in neuroendocrine neoplasias. Surgery, 154(6): pp. 1185-92; discussion 1192-3 [DOI] [PubMed]
- 100.Soldevilla B et al (2021) Comprehensive plasma metabolomic profile of patients with advanced neuroendocrine tumors (NETs). Diagnostic and biological relevance. Cancers (Basel), 13(11) [DOI] [PMC free article] [PubMed]
- 101.La Salvia A et al (2024) Metabolomic profile of neuroendocrine tumors identifies methionine, porphyrin, and Tryptophan metabolisms as key dysregulated pathways associated with patient survival. Eur J Endocrinol 190(1):62–74 [DOI] [PubMed] [Google Scholar]
- 102.Jannin A et al (2023) Metabolism of pancreatic neuroendocrine tumors: what can omics tell Us?? Front Endocrinol (Lausanne) 14:1248575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hu C et al (2024) FOXA2-initiated transcriptional activation of INHBA induced by methylmalonic acid promotes pancreatic neuroendocrine neoplasm progression. Cell Mol Life Sci 81(1):50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.López-López Á et al (2020) Oxidized lipids in the metabolic profiling of neuroendocrine tumors - Analytical challenges and biological implications. J Chromatogr A,: p. 1625 [DOI] [PubMed]
- 105.Modica R (2024) Dyslipidemia, lipid-lowering agents and neuroendocrine neoplasms: new horizons. Endocrine 85(2):520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kawasaki K et al (2020) An organoid biobank of neuroendocrine neoplasms enables Genotype-Phenotype mapping. Cell 183(5):1420–1435e21 [DOI] [PubMed] [Google Scholar]
- 107.Detjen K et al (2021) Models of gastroenteropancreatic neuroendocrine neoplasms: current status and future directions. Neuroendocrinology 111(3):217–236 [DOI] [PubMed] [Google Scholar]
- 108.Chamberlain CE et al (2018) A Patient-derived xenograft model of pancreatic neuroendocrine tumors identifies sapanisertib as a possible new treatment for Everolimus-resistant tumors. Mol Cancer Ther 17(12):2702–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Z et al (2024) Molecular subtypes of neuroendocrine carcinomas: A cross-tissue classification framework based on five transcriptional regulators. Cancer Cell 42(6):1106–1125e8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.