Abstract
Using spectral-domain optical coherence tomography (SD-OCT) in patients with diabetic macular edema (DME), we studied the relationships between consecutive aflibercept treatments, soluble CD14 (sCD14) in the aqueous humor (AH), and anatomical features, including hyper-reflective foci (HFs) and other morphologic characteristics. This prospective study included 23 eyes of 23 patients with DME treated with five consecutive monthly intravitreal aflibercept injections. At each visit, sCD14 and VEGF levels in the AH were measured using ELISA. The number of HFs, central macular thickness (CMT), and volume of intraretinal fluid (IRF) and subretinal fluid (SRF) were also assessed. One month after fifth injections, there were significant decreases in CMT, the number of HFs, and the volumes of IRF and SRF. The level of sCD14 also decreased. Although sCD14 levels showed a downward trend, the change was not statistically significant. The group with reduced sCD14 exhibited improvements in all the factors, including visual acuity. In contrast, the group with increased sCD14 only showed significant decreases in CMT. When the data were categorized into two groups based on the final visual outcome, patients with good final visual acuity had consistently lower levels of sCD14 at all visits. This study highlights sCD14 as a potential biomarker for assessing treatment response to aflibercept in DME.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-93472-w.
Subject terms: Macular degeneration, Predictive markers
Introduction
The global incidence of adult diabetes in 2021 was estimated to be 10.5% (536.6 million people), with a projected increase to 12.2% (783.2 million) by 20451. Diabetic retinopathy (DR), a major complication of diabetes, remains the leading cause of blindness. Although the pathogenesis of DR is not fully understood, inflammation—encompassing monocyte/macrophage recruitment and microglial activation—compromises the blood–retinal barrier2. Diabetic macular edema (DME), a primary cause of vision loss in DR, is characterized by intraretinal edema due to vascular permeability and elevated vascular endothelial growth factor (VEGF)3.
DR and DME are now considered to be consequences of neurovascular unit (NVU) dysfunction, involving early low-grade neuroinflammation4,5. In line with this, research efforts have shifted toward exploring a broader spectrum of inflammatory mediators and cytokines beyond VEGF to better understand the inflammatory cascades contributing to DME6–8. Among these, the role of microglia and related neuroinflammatory pathways has drawn particular interest, prompting investigations into various innate immune biomarkers9,10. CD14, a cytokine predominantly expressed by microglia, monocytes, and macrophages11, exists in two forms: a membrane-bound form, which is associated with cellular signaling, and a soluble form (sCD14), which can act as a mediator of systemic inflammation.
In DME, sCD14 levels are elevated in the aqueous humor (AH) and vitreous humor, showing a strong association with increased VEGF levels12. This relationship highlights the potential role of sCD14 as a regulator of VEGF-driven angiogenesis and vascular permeability12. Evidence from previous studies, including our own, has demonstrated that sCD14 is not merely a byproduct of inflammation but may play an active role in the pathogenesis of DR12. Of particular note, hyperreflective foci (HFs), identified via spectral-domain optical coherence tomography (SD-OCT), are increasingly recognized as potential biomarkers of DME activity13. Although the precise origin of HFs remains debated, prevailing theories attribute their formation to activated microglia or lipoprotein exudation14. Our previous study demonstrated that elevated sCD14 levels in the AH of patients with diffuse edema are accompanied by a higher prevalence of HFs in the inner retina compared to patients with focal edema. This finding underscores the association between sCD14 levels and HF density, suggesting that HFs may reflect the severity of retinal inflammation mediated by activated microglia15.
Current treatments for DME primarily include anti-VEGF agents, such as ranibizumab, aflibercept, brolucizumab, and faricimab, along with intravitreal steroids. Among these, aflibercept is particularly favored for its prolonged duration of action, as validated in the VIVID and VISTA trials16. Aflibercept functions as a soluble decoy receptor, binding VEGF-A, VEGF-B, and placental growth factor (PIGF), thereby addressing both angiogenic and neuroinflammatory pathways. Despite the proven efficacy of aflibercept in reducing retinal fluid and improving visual outcomes, research examining its effects on neuroinflammatory changes, particularly those involving microglia-related biomarkers such as sCD14 and HFs, remains limited. Additionally, the relationship between these biomarkers and fluid compartments in DME patients warrants further investigation.
To address this gap, we conducted a prospective study employing a standardized protocol of five consecutive aflibercept injections, consistent with the VIVID and VISTA trial conditions. By quantitatively assessing biomarkers such as AH sCD14 levels, HFs, and fluid compartments via SD-OCT, this study aimed to comprehensively evaluate the anti-inflammatory potential of aflibercept. Furthermore, we sought to identify factors contributing to variability in individual treatment responses, potentially linked to neuroinflammatory processes.
Methods
Study design
The FORESIGHT study was a prospective, open-label, investigator-initiated, single-arm clinical trial conducted at Konkuk University Medical Center, Seoul, Korea. The study adhered to the Declaration of Helsinki and received approval from the institutional review board of Konkuk University Medical Center (approval number: 1100065). Written informed consent was obtained from all participants.
Eligibility criteria aligned with those of the VISTA and VIVID studies, which established the efficacy and safety of intravitreal aflibercept (2.0 mg) for treating DME. The study was registered with the Clinical Research Information Service (CRIS), Korea (registration number: KCT0003712, 03/04/2019). The first participant was enrolled on 17/05/2019.
The inclusion criteria were similar to those of the VIVID/VISTA study. Detailed information can be found in Supplementary Data 1. All patients underwent a complete ophthalmic examination, including BCVA testing using ETDRS charts, intraocular pressure (IOP) measurement using a pneumatic tonometer (CANON TX-F, Canon, Inc., Tokyo, Japan), slit-lamp biomicroscopy, fundus photography and spectral domain OCT (Spectralis HRA + OCT, Heidelberg Engineering, Inc., Heidelberg, Germany) at baseline and during follow-up. Fluorescein angiography (FA) and indocyanine green angiography (ICGA) were performed at baseline using confocal scanning laser ophthalmoscopy (HRA; Heidelberg Engineering, Inc., Heidelberg, Germany). The full clinical trial protocol has been uploaded in the ‘Related Manuscript Files’ section.
Treatment and quantification of the levels of sCD14 and VEGF
Patients received five monthly intravitreal aflibercept injections (2 mg/0.05 ml). Before each injection, 0.1–0.2 ml of AH was obtained via anterior chamber paracentesis. Levels of sCD14 and VEGF in AH samples were measured using multiplex ELISA (R&D Systems, Minneapolis, MN, USA). Signal intensity was quantified using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). Each assay was performed in duplicate to ensure reliability.
Quantitative measurement of HF density via SD-OCT
Horizontal high-resolution SD-OCT images were obtained every 4 weeks from baseline to week 20. HFs were manually quantified by an ophthalmology resident (N.K.), following the method described by Midena et al.17 We performed a semiautomated analysis using ImageJ software (V.1.53; National Institutes of Health, Bethesda, Maryland, USA): (1) OCT images were imported into ImageJ and converted to an 8-bit format. (2) A denoising filter (A_trous_filter.java) was applied (parameters: k1 = 4.0; k2 = 3.0; k3 = 3.0; k4 = 0.0; k5 = 0.0). (3) The contrast was enhanced using the CLAHE tool (block size = 127; histogram bins = 256; slope = 3.00). (4) Regions of interest, spanning the retinal nerve fiber layer to the external limiting membrane, were manually delineated using the Freehand Selection tool. (5)
HFs were counted using the Spot Counter plugin of ImageJ. The results were independently validated by a retinal specialist (H.L.). Discrepancies were resolved by consensus (Supplementary Fig. 1).
Volume measurement of the fluid compartment
A volume scan comprising 49 horizontal B-scans covering a 9 × 6-mm area of the macular region centered on the fovea was acquired using SD-OCT at every visit. Based on our previous study, all the IRF and SRF in the volume scans were segmented and quantified using U-Net algorithms18. Automatic segmentations were reviewed and corrected as needed by two retinal specialists (H.L. and H.C.). Fluid volumes were calculated by multiplying segmented areas from each B-scan by the inter-scan distance (123 μm). Results were converted into cubic millimeters for analysis (Supplementary Fig. 2).
Statistical analysis
All statistical analyses were performed using IBM SPSS version 22 (IBM Corp., Chicago, IL, USA). Continuous variables across multiple visits were compared using mixed-model analysis or repeated-measures ANOVA when no data were missing. Parameters included BCVA (ETDRS), cytokine levels (sCD14, VEGF), and structural factors (CMT, HFs, IRF, and SRF). Multivariate regression analyses were adjusted for age and sex. Pearson correlation coefficients were calculated to evaluate relationships between parameters. A significance threshold of p < 0.05 was applied. Figures were generated using GraphPad Prism v.9.0 (Dotmatics, San Diego, CA, USA).
Results
Baseline demographic data
Twenty-four patients were initially enrolled in the study; however, one patient was excluded due to a needle puncture of the anterior lens capsule during AH acquisition, which was classified as an adverse event. The baseline characteristics of the remaining participants are shown in Table 1. The mean age was 57.7 years, the mean duration of diabetes was 12.48 years, and the mean hemoglobin A1c (HbA1c) level was 7.69%.
Table 1.
Baseline characteristics.
| Parameters | Value |
|---|---|
| Number of eyes, No. | 23 |
| Sex (M/F), No. (%) | 13 (56.5%)/10 (43.5%) |
| Age, y (SD) | 57.70 (7.03) |
| DM duration, y (SD) | 12.48 (9.49) |
| HbA1c, % (SD) | 7.69 (1.41) |
| Total cholesterol | 166.30 (31.64) |
| HDL | 60.57 (21.63) |
| LDL | 82.26 (31.88) |
| TG | 112.26 (57.98) |
HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride.
Overall treatment outcome
One month after five consecutive intravitreal injections, the majority of patients demonstrated significant improvements in several key parameters. Specifically, CMT, the number of HFs, and the volumes of IRF and SRF all showed significant reductions, accompanied by a marked improvement in visual acuity as measured by ETDRS charts. Aqueous VEGF levels also decreased following treatment. However, although sCD14 levels displayed a downward trend, the change was not statistically significant (Supplementary Table 1, Fig. 1).
Fig. 1.
Graphs illustrating the overall treatment effects of five consecutive aflibercept injections. The quantified levels of the investigated parameters at baseline and at the final visit are presented. Asterisks denote a p-value less than 0.05.
Comparison between patients with increased and decreased sCD14
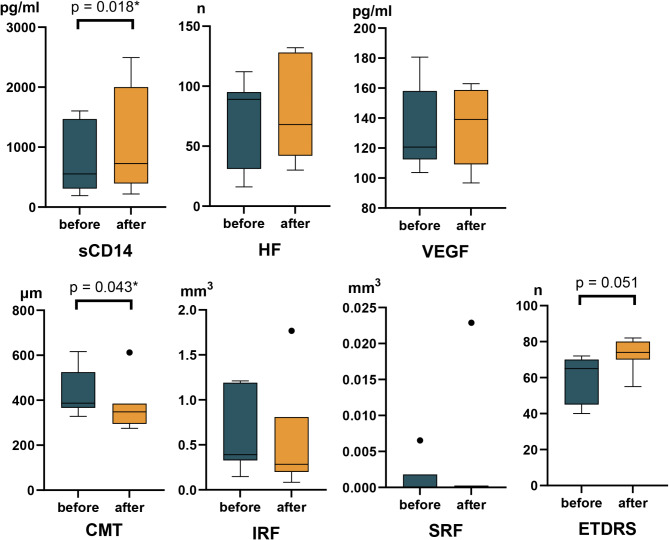
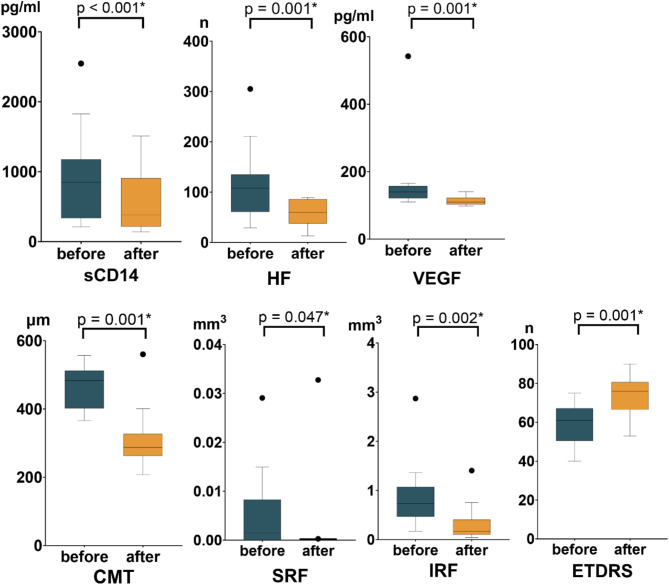
Among the 23 patients who completed the study, 7 exhibited increased sCD14 levels post-treatment, while the remaining 16 showed decreased levels. In the increased sCD14 group, no parameters other than CMT improved significantly after treatment (Fig. 2, Supplementary Table 2). Conversely, patients in the decreased sCD14 group experienced significant improvements in all assessed parameters, including ETDRS visual acuity, CMT, HFs, and fluid volumes (Fig. 3, Supplementary Table 3).
Fig. 2.
Graphs illustrating the treatment effects in the group with increased sCD14. Box-whisker graphs of each parameter at baseline and the final visit. Box-whisker plots showing the 25th and 75th percentile ranges (boxes) with 95% Tukey’s confidence intervals (whiskers) and median values (transverse lines in the box). Asterisks denote a p-value less than 0.05.
Fig. 3.
Graphs illustrating the treatment effects in the group with decreased sCD14. Box-whisker plots of each parameter at baseline and at the final visit; box-whisker plots showing the 25th and 75th percentile ranges (boxes) with 95% Tukey’s confidence intervals (whiskers) and median values (transverse lines in the box); asterisks denote p values less than 0.05.
Factors potentially influencing changes in sCD14, including age, HbA1c levels, diabetes duration, and cholesterol levels (total cholesterol, HDL, LDL, and triglycerides), were analyzed. However, no significant differences were found between the groups. Representative cases of patients with increased and decreased sCD14 levels are shown in Fig. 4.
Fig. 4.
Representative cases. (A) Decreased sCD14 group—In a 34-year-old female, the ETDRS increased from 62 (Snellen 0.3) before injection to 73 (Snellen 0.8) after 5 intravitreal injections, and the HFs decreased from 142 to 72, indicating a positive correlation with sCD14. The VEGF levels did not significantly change (increase from 120.68 pg/ml to 122.61 pg/ml), suggesting that inflammatory factors other than VEGF had an effect. (B) Increased sCD14 group—In a 66-year-old woman, the ETDRS decreased from 59 (Snellen 0.3) before injection to 55 (Snellen 0.3) after 5 intravitreal injections, and the HFs increased from 89 to 132, indicating a positive correlation with sCD14; VEGF levels did not significantly change (decrease from 103.69 pg/ml to 96.72 pg/ml).
Longitudinal analysis of visual acuity subgroups
Patients were categorized into “good” and “poor” final visual outcome groups based on their visual acuity at the final visit. The good outcome group comprised the top 12 patients, while the poor outcome group consisted of the remaining 11 patients. Both groups demonstrated improvements in vision following five aflibercept injections. Across all time points, the good outcome group tended to have lower levels of sCD14, HFs, CMT, IRF, and SRF compared to the poor outcome group; however, these differences were not statistically significant (Supplementary Fig. 3).
Correlation of sCD14 changes with other parameters
Correlation analysis revealed that changes in sCD14 were negatively associated with the initial number of HFs (ρ = -0.545) and positively associated with the total change in HFs (ρ = 0.693) (Supplementary Table 4). Changes in sCD14 also demonstrated positive correlations with changes in CMT, VEGF, and IRF volumes.
Discussion
This study has strengths in that its design was prospective, and all relevant clinical information, including the visual acuity, quantified fluid compartments, number of HFs in OCT images, and sCD14 levels, were collected at all five consecutive time points with monthly aflibercept injections. In addition, this study reports the results of five consecutive intravitreal injections of aflibercept, a widely used and highly effective treatment for DME, in a real-world clinical setting. Moreover, quantification of fluid compartments and the number of HFs was performed using a semiautomated method, minimizing grader bias as much as possible.
Several landmark studies on the treatment of DME with aflibercept exist. In the DA VINCI study, an early clinical trial evaluating the efficacy of aflibercept in DME, aflibercept was initiated with three monthly loading doses and then administered as needed (PRN; Pro Re Nata); the results were consistent with those obtained after the administration of aflibercept every 4 weeks19. According to the one-year results of protocol T, six monthly loading doses followed by PRN improved the mean visual acuity letter score from baseline to one year by 13.3 letters20. The VIVID and VISTA are the most representative phase III clinical trials that have confirmed the effectiveness and safety of aflibercept administered for 148 weeks in 872 DME patients. Compared with those after laser photocoagulation, significantly better functional and anatomical outcomes were demonstrated with aflibercept administration every four or eight weeks after five initial monthly injections16. In addition, the efficacy of 5 consecutive injections of aflibercept for treating DME was further confirmed in other studies4,16,19–23. In the present study, we evaluated the efficacy of five consecutive aflibercept injections and found decreases in CMT, IRF, SRF, and HF counts on SD-OCT and in VEGF levels in the AH in 23 eyes of DME patients, ultimately leading to an improvement in visual acuity.
Umazume et al.12 reported significantly greater levels of sCD14 and VEGF in vitreous fluid and AH in patients with DME than in nondiabetic controls. It has also been reported that sCD14 levels are increased in the vitreous fluid of patients with PDR24. Previously, we demonstrated that sCD14 levels in the AH were greater in patients with DME than in control subjects, and this phenomenon was more evident in patients with diffuse edema than in those with focal edema15. Consistent with our previous study, this study also revealed an increase in HFs in the group with increased sCD14 and a decrease in HFs in the group with decreased sCD14. Correlation analysis between other factors and the change in sCD14 concentration confirmed that the change in the number of HFs had the highest correlation coefficient (0.693). It was also the most significant, with a p-value of less than 0.001 (Supplementary Table 4).
Although all the parameters, including vision, improved in this study, the sCD14 levels did not significantly differ before and after five consecutive treatments. To explain this unexpected result, we divided the study group into two subgroups: one with increased sCD14 levels and the other with decreased sCD14 levels. Alternatively, the division was based on the degree of final visual acuity. Our study, through the analysis of two subgroups, was able to determine whether sCD14 levels could serve as an indicator of DME severity. The group with decreased sCD14 at the final visit exhibited better outcomes, as indicated by significant decreases in VEGF, HFs, CMT, IRF, and SRF. The patients in the group also exhibited increased visual acuity (Fig. 3), and all anatomical factors, including HFs, CMT, IRF, and SRF, showed a positive correlation with the change in sCD14 (Supplementary Table 4). As described earlier, DME is thought to result from neuroinflammation accompanied by vascular leakage involving complex immune responses, such as changes in cytokines and retinal activation of multiple inflammatory cells, including microglia12,25. Therefore, in the decreased sCD14 group, the neuroinflammatory response was well controlled throughout the visits26. Unlike other anti-VEGF agents, aflibercept traps not only VEGF but also PlGF; its action may constitute an optimized strategy for targeting neuroinflammation in DME patients because PlGF modulates inflammation and induces the chemotaxis of monocytes and macrophages; therefore, aflibercept concomitantly reduces sCD14, HFs, and fluid compartments, resulting in an increase in VA27. On the other hand, in the group with increased sCD14, changes in VA and all anatomical parameters other than CMT were found to be insignificant. This finding indicates resistance to aflibercept (Fig. 2). Therefore, refractory sCD14 levels might be a significant factor indicating aflibercept resistance. However, further studies are needed to determine whether high-dose aflibercept (8 mg) could provide additional therapeutic benefits to patients with nonreducing sCD14.
When the patients were categorized into two groups based on their final visual outcome, the patients with poor visual acuity had a higher level of sCD14, particularly from baseline to visit 4, than did patients with better visual outcomes (Fig. 2); however, this difference was not statistically significant. During follow-up, the well-known biomarkers of visual prognosis in DME patients, such as HFs and fluid compartments, including IRF and SRF, converged to similar levels in both groups: those with good and poor final visual acuity. However, patients in the group with good final visual acuity maintained a lower level of CMT than patients with poor final visual acuity. These results suggest that five consecutive aflibercept treatments effectively improved HF counts, IRF, and SRF, regardless of the visual outcome. Additionally, CMT remained greater in the group with poor visual outcomes than in the group with good visual outcomes. Therefore, the sustained increase in sCD14 and CMT before visit 4 could be a reliable biomarker for poor visual outcomes. Moreover, the increase in sCD14 and CMT at all visits may indicate an association between sCD14 and retinal thickening accompanied by changes in IRF or SRF, as demonstrated in our previous study15.
This study has several limitations. First, the number of included patients was relatively small, and future studies with larger cohorts are necessary to validate and strengthen our findings. Second, we were unable to directly confirm microglial activation in eyes with higher sCD14 levels or to establish definitively that hyperreflective foci (HFs) observed on SD-OCT represent activated microglia. Further immunohistological research is needed to clarify the relationship between increased sCD14 levels, microglial activation, and HFs in DME. Third, while we examined several systemic and local factors potentially influencing sCD14 changes, we did not specifically investigate the duration of macular edema or thoroughly evaluate systemic conditions such as hypertension or renal dysfunction in relation to non-responsiveness. Furthermore, although patients with ischemic maculopathy were excluded, a more comprehensive assessment of local retinal conditions may be necessary. Finally, improved methods for quantifying HFs and retinal fluid compartments would enhance the accuracy and objectivity of related analyses.
In conclusion, although changes in sCD14 levels did not reach statistical significance in this study, subgroup analysis revealed that decreased sCD14 levels were associated with significant improvements in multiple anatomical and functional parameters. These findings underscore the potential of sCD14 as a meaningful biomarker in DME and highlight the need for further research with larger cohorts to confirm these observations and clarify the role of cytokines in retinal neuroinflammation and treatment outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
H. L. and M.L. have full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analyses; Concept and design: H. C.; Data acquisition, analysis, or interpretation; Statistical analysis: N.K. and D.M.; Drafting of the manuscript: H.L., M.L., N.K., C.S., and H. C.; Critical revision of the manuscript: All authors. H.L. and M.L. contributed equally to this study.
Funding
This study was supported by the National Research Foundation of Korea and funded by the Ministry of Science and ICT (RS-2020-NR046274), the Ministry of Health and Welfare of the Republic of Korea (RS-2022-KH128782), and the Investigator Initiated Research Fund from Bayer Korea. The sponsor or funding organization played no role in the design or conduct of this research.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to our hospital’s policy regarding patient records but are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hyungwoo Lee and Minsub Lee contributed equally to this work.
References
- 1.Sun, H. et al. IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract.183, 109119. 10.1016/j.diabres.2021.109119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omri, S. et al. Microglia/macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: Role of PKCzeta in the Goto Kakizaki rat model. Am. J. Pathol.179, 942–953. 10.1016/j.ajpath.2011.04.018 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tatsumi, T. Current treatments for diabetic macular edema. Int. J. Mol. Sci.24, 9591. 10.3390/ijms24119591 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroder, S., Palinski, W. & Schmid-Schonbein, G. W. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am. J. Pathol.139, 81–100 (1991). [PMC free article] [PubMed] [Google Scholar]
- 5.Bianco, L. et al. Neuroinflammation and neurodegeneration in diabetic retinopathy. Front. Aging Neurosci.14, 937999. 10.3389/fnagi.2022.937999 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiraya, T. et al. Aqueous cytokine levels are associated with reduced macular thickness after intravitreal Ranibizumab for diabetic macular edema. PLoS One12, e0174340. 10.1371/journal.pone.0174340 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung, Y. R. et al. Role of inflammation in classification of diabetic macular edema by optical coherence tomography. J. Diabetes Res.2019, 8164250. 10.1155/2019/8164250 (2019). [DOI] [PMC free article] [PubMed]
- 8.Noma, H., Yasuda, K. & Shimura, M. Involvement of cytokines in the pathogenesis of diabetic macular edema. Int. J. Mol. Sci.22, 3427. 10.3390/ijms22073427 (2021). [DOI] [PMC free article] [PubMed]
- 9.Kinuthia, U. M., Wolf, A. & Langmann, T. Microglia and inflammatory responses in diabetic retinopathy. Front. Immunol.11, 564077. 10.3389/fimmu.2020.564077 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan, L., Zhang, L., Liu, X., Li, S. & Zou, J. J. M. Identification of differential immune cells and related diagnostic genes in patients with diabetic retinopathy. Medicine102, e35331 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landmann, R., Muller, B. & Zimmerli, W. CD14, new aspects of ligand and signal diversity. Microb. Infect.2, 295–304. 10.1016/s1286-4579(00)00298-7 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Umazume, K. et al. Effects of soluble CD14 and cytokine levels on diabetic macular edema and visual acuity. Retina33, 1020–1025. 10.1097/IAE.0b013e31826f0688 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Coscas, G. et al. Hyperreflective dots: A new spectral-domain optical coherence tomography entity for follow-up and prognosis in exudative age-related macular degeneration. Ophthalmologica229, 32–37. 10.1159/000342159 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Bolz, M. et al. Optical coherence tomographic hyperreflective foci: A morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology116, 914–920. 10.1016/j.ophtha.2008.12.039 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Lee, H., Jang, H., Choi, Y. A., Kim, H. C. & Chung, H. Association between soluble CD14 in the aqueous humor and hyperreflective foci on optical coherence tomography in patients with diabetic macular edema. Invest. Ophthalmol. Vis. Sci.59, 715–721. 10.1167/iovs.17-23042 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Heier, J. S. et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology123, 2376–2385. 10.1016/j.ophtha.2016.07.032 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Midena, E. et al. OCT hyperreflective retinal foci in diabetic retinopathy: A semi-automatic detection comparative study. Front. Immunol.12, 613051. 10.3389/fimmu.2021.613051 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, H., Kang, K. E., Chung, H. & Kim, H. C. Automated segmentation of lesions including subretinal hyperreflective material in neovascular age-related macular degeneration. Am. J. Ophthalmol.191, 64–75. 10.1016/j.ajo.2018.04.007 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Do, D. V. et al. The DA VINCI study: Phase 2 primary results of VEGF trap-eye in patients with diabetic macular edema. Ophthalmology118, 1819–1826. 10.1016/j.ophtha.2011.02.018 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Diabetic Retinopathy Clinical Research, N. et al. Aflibercept, bevacizumab, or Ranibizumab for diabetic macular edema. N. Engl. J. Med.372, 1193–1203. 10.1056/NEJMoa1414264 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pak, K. Y. et al. One-year results of treatment of diabetic macular edema with aflibercept using the treat-and-extend dosing regimen: The VIBIM study. Ophthalmologica243, 255–262. 10.1159/000504753 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Han, Y. E., Jo, J., Kim, Y. J. & Lee, J. Factors affecting intensive Aflibercept treatment response in diabetic macular edema: A real-world study. J. Diabetes Res.2023, 1485059. 10.1155/2023/1485059 (2023). [DOI] [PMC free article] [PubMed]
- 23.Chung, Y. R., Lee, K. H. & Lee, K. Clinical application of intravitreal Aflibercept injection for diabetic macular edema comparing two loading regimens. Med. (Kaunas)59. 10.3390/medicina59030558 (2023). [DOI] [PMC free article] [PubMed]
- 24.Hernandez, C. et al. Lipopolysaccharide-binding protein and soluble CD14 in the vitreous fluid of patients with proliferative diabetic retinopathy. Retina30, 345–352. 10.1097/iae.0b013e3181b7738b (2010). [DOI] [PubMed] [Google Scholar]
- 25.Yu, Y., Chen, H. & Su, S. B. Neuroinflammatory responses in diabetic retinopathy. J. Neuroinflamm.12, 141. 10.1186/s12974-015-0368-7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan, W. W., Lin, F. & Fort, P. E. The innate immune system in diabetic retinopathy. Prog. Retin. Eye Res.84, 100940. 10.1016/j.preteyeres.2021.100940 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starace, V. et al. The role of inflammation and neurodegeneration in diabetic macular edema. Ther. Adv. Ophthalmol.13, 25158414211055963. 10.1177/25158414211055963 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to our hospital’s policy regarding patient records but are available from the corresponding author upon reasonable request.