Abstract
The Vibrio fischeri—Euprymna scolopes symbiosis has become a powerful animal—microbe model system to examine the genetic underpinnings of symbiont development and regulation. Although there has been a number of elegant bacterial genetic technologies developed to examine this symbiosis, there is still a need to develop more sophisticated methodologies to better understand complex regulatory pathways that lie within the association. Therefore, we have developed a suite of CRISPR interference (CRISPRi) vectors for inducible repression of specific V. fischeri genes associated with symbiotic competence. The suite utilizes both Tn7-integrating and shuttle vector plasmids that allow for inducible expression of CRISPRi dCas9 protein along with single-guide RNAs (sgRNA) modules. We validated this CRISPRi tool suite by targeting both exogenous (an introduced mRFP reporter) and endogenous genes (luxC in the bioluminescence producing lux operon, and flrA, the major regulatory gene controlling flagella production). The suite includes shuttle vectors expressing both single and multiple sgRNAs complementary to the non-template strand of multiple targeted genetic loci, which were effective in inducible gene repression, with significant reductions in targeted gene expression levels. V. fischeri cells harboring a version of this system targeting the luxC gene and suppressing the production of luminescence were used to experimentally validate the hypothesis that continuous luminescence must be produced by the symbiont in order to maintain the symbiosis at time points longer than the known 24-h limit. This robust new CRISPRi genetic toolset has broad utility and will enhance the study of V. fischeri genes, bypassing the need for gene disruptions by standard techniques of allelic knockout-complementation-exchange and the ability to visualize symbiotic regulation in vivo.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00203-025-04354-8.
Keywords: CRISPRi, Symbiosis, Vibrio, Squid, dCas9
Introduction
The bioluminescent marine bacterium Vibrio fischeri is a widely adopted model organism for studies of symbiosis (with its host squid Euprymna scolopes) (Stabb and Visick 2014), biofilm formation (Yildiz and Visick 2009; Visick 2009), bioluminescence (Septer and Stabb 2012), and quorum sensing (Verma and Miyashiro 2013). Research in these fields has been increasingly facilitated by the development of progressively more sophisticated genetic tools to manipulate the V. fischeri genome. Genetic modification tools were first adapted for use in V. fischeri in 1992 with the demonstration of homologous insertional mutagenesis from conjugatable plasmid vectors (Dunlap and Kuo 1992). Motility was identified as the first V. fischeri phenotypic characteristic required for successful host colonization using a Mu dI 1681 transposon plasmid vector for random mutagenesis in 1994 (Graf et al. 1994). Tools for Tn7 transposon targeted insertional mutagenesis in V. fischeri were first demonstrated in 1997 (Visick and Ruby 1997). Development of both stable and suicide plasmid vectors (Dunn et al. 2006) further enhanced the ability to probe V. fischeri genetics. Subsequently, natural transformation based genetic modification tools using the forced over-expression of the natural transformation master regulator tfoX were adapted for use in homologous recombination based insertion/deletion/allelic exchange methods in V. fischeri (Pollack-Berti, et al. 2010). Further work enhanced the utility (Visick and Ruby 1997), and transformation efficiencies (Fidopiastis et al. 2024) of induced natural transformation.
Methods to probe gene function through the controlled modulation of expression of a targeted gene were also developing and being adapted for use in bacteria, including V. fischeri. RNA interference methods, using plasmids vectors with inducible promoter expression of antisense RNA that is complementary to the mRNA of a targeted gene. This has been shown to repress gene expression, from mild to knock-out levels (Magistro et al. 2018), and also target multiple genes (Nakashima et al. 2012). Another use of inducible promoters to modulate gene expression in a titratable and reversable manner involved a Tn5 transposon genetic tool that was adapted for use in V. fischeri and used in the construction of strains in which IPTG-inducible promoters were randomly inserted into the genome to identify and control the expression of genes controlling phenotypic characteristics (Ondrey and Visick 2014).
While inducible control of gene expression by promoter replacement is a valuable tool to link gene to phenotype (Judson and Mekalanos 2000; Ondrey and Visick 2014) endogenous promoter replacement with non-endogenous inducible promoters may not allow for the normal range of expression of the inducible target gene. An alternative approach which retains the normal range of endogenous gene expression is CRISPRi (clustered regularly interspaced short palindromic repeats interference) repression of native gene expression (Qi et al. 2013). In CRISPRi, a mutated de-active (no nuclease function) form of the Cas9 protein (dCas9), in conjunction with a programable sgRNA guide, induces steric hindrance of RNA polymerase promoter binding and transcript expression leading to repressed protein expression (Qi et al. 2013). By using an inducible promoter, dCas9 promoted gene repression can be reversibly induced and relieved to normal physiologic levels in a temporal and spatial manner (Banta et al. 2020a, b). The sgRNA will usually target the promoter or 5’-end of a transcribed gene via complementary binding of the spacer sequence (20-nt for the commonly used SpdCas9 (from Streptococcus pyogenes) (Geyman et al. 2024; Qi et al. 2013). A constraint that limits the targetability of CRISPRi systems is that the targeted sequence must be located next to a protospacer adjacent motif (PAM) sequence (5′-NGG-3′ for S. pyogenes dcas9) (Didovyk et al. 2016), which means that true single base pair targeting of a given genetic loci is not yet possible, though the hunt is on for new PAM-less cas9 variants (Collias and Beisel 2021). Other limitations of CRISPRi include dcas9 toxicity that has been observed in some bacterial species at high dcas9 expression levels (Cui et al. 2018), and the possibility of off-target effects, where the dcas9-sgRNA complex binds to unintended DNA sequences that share partial homology to the intended target (Feng et al. 2021). Strategies to mitigate off-target effects include off-target detection, with several in silico tools, such as CRISPy-web (CRISPy-web), CRISPR Design Tool (synthego.com/products/bioinformatics/crispr-design-tool), and FlashFry (Naeem et al. 2020), which can identify gene specific potential PAM-adjacent 20-nt targeting sequences while also scanning the bacterial genome for potential off-target sequences with high homology. Locations of potential targeting sequences are ranked by the number of potential off-target sites found and their degree of homology (Naeem et al. 2020). It has been observed that when CRISPRi is targeted to a gene within an operon (a group of genes that are transcribed together as a polycistronic transcript), downstream genes in the operon are also repressed (Peters et al. 2016; Qi et al. 2013). This polar effect can make it challenging to accurately identify the function of individual genes within an operon. However, it can also be an advantage, as operons often contain genes involved in the same biological pathway, so silencing one gene can lead to the silencing of related genes.
The use of bacterial CRISPRi has been expanding, with applications ranging from the elucidation of gene functions and the screening of essential genes (Todor et al. 2021), to diverse projects in synthetic biology and metabolic engineering (Didovyk et al. 2016). CRISPRi systems have been adapted for use in diverse bacterial species, (Peters et al. 2019), (Banta et al. 2020a, b; Enright et al. 2024; Geyman et al. 2024), including members of the Vibrionaceae such as Vibrio cholera (Caro et al. 2019), Vibrio natriegens (Lee et al. 2019), Vibrio casei (Peters et al. 2019), and Vibrio parahemolyticus (Jiang et al. 2024). Recently the efficacy of the Mobile-CRISPRi Tn7 vector (Peters et al. 2019) for the targeted IPTG-inducible repression of a chromosomally integrated GFP reporter gene as well as the essential gene rpoB was demonstrated in V. fischeri (Geyman et al. 2024). When induced in culture, the chromosomally integrated dcas9/sgRNA Mobile-CRISPRi system significantly reduced reporter GFP expression levels and introduced a significant growth lag, demonstrating the utility of this system for targeted gene repression in V. fischeri.
In this work we have extended the utility of an inducible CRISPRi system in V. fischeri by incorporating a plasmid vector containing a multiplexed sgRNA expression cassette with three independently targetable sgRNA moieties within a nonrepetitive extra-long sgRNA array (Reis et al. 2019) for use with the Tn7 integrating IPTG-inducible (PLlac0-1 promoter) dcas9 expression plasmid vector pJMP1189, one of the Mobile-CRISPRi suite of vectors (Peters et al. 2019). Our V. fischeri CRISPRi system demonstrates inducible, titratable, and reversible repression of the expression of single or multiple targeted V. fischeri gene(s) in culture. The design, generation, and functional testing of our V. fischeri CRISPRi system in vitro, and its application in experiments probing the temporal monitoring of bioluminescence during the squid-Vibrio symbiosis establishment are described below.
Results
Functional testing of CRISPRi repression of mRFP gene expression
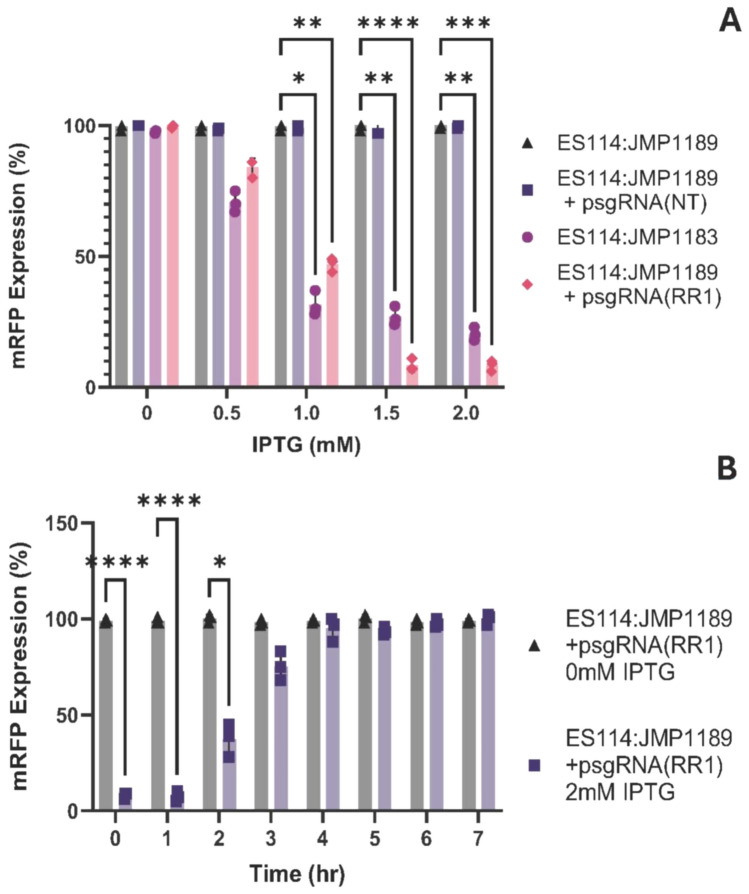
To quantitatively test the gene repression capability of the PLlac0-1 driven dcas9 and sgRNA cassettes, we initially assayed the repression of mRFP fluorescence from the ES114:JMP1183 strain, which has both cassettes integrated at the attTn7 site along with a constitutive mRFP reporter gene. We concurrently assayed mRFP repression in the ES114:JMP1189 strain (which is isogenic to strain ES114:JMP1183 but without a sgRNA cassette) carrying a PLlac0-1 driven psgRNA (RR1) expression plasmid, and the psgRNA(NT) expression plasmid which has a 20-nt spacer designed to not match anywhere in the V. fischeri genome (Figs. 1 and 2). Both the genome integrated and plasmid borne sgRNA cassettes have the same 20-nt spacer (RR1) targeting the integrated constitutive mRFP reporter.
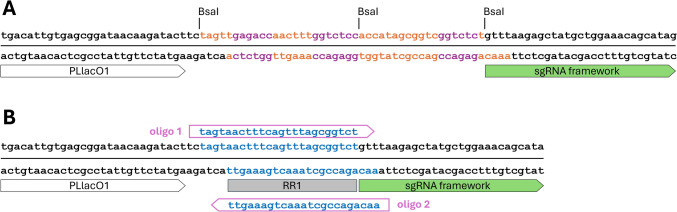
Fig. 1.
SgRNA spacer cloning. A the triple BsaI TIIS cloning site allows for efficient cloning of 20-nt spacer sequences in-frame with the adjacent sgRNA framework sequence. B Ligation of annealed 20-nt targeting oligos into the resulting empty BsaI-cut cloning site. The spacer shown is the (RR1) spacer used in this study to target the mRFP gene
Fig. 2.
A Titratable repression of mRFP. mRFP expression was calculated as specific fluorescence (relative fluorescence units/OD600) of induced strains normalized to the control strain ES114:JMP1189. Values reported reflect the mRFP repression at mid-log growth (OD600 = 0.4). All values reported are mean values and error bars reflect the standard deviation from the mean. Statistics were done using a two-way ANOVA, with Šídák’s multiple comparisons test *: p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. B Reversal of mRFP repression. mRFP expression was calculated as specific fluorescence (relative fluorescence units/OD600) of the initially IPTG repressed ES114:JMP1189/psgRNA(RR1) strain normalized to the unrepressed ES114:JMP1189/psgRNA(RR1) control strain. Values reported reflect the mRFP repression at mid-log growth (OD600 = 0.4). Each point reflects the mean of 3 samples with their respective standard deviation from the mean. Statistics were done using a two-way ANOVA, with Šídák’s multiple comparisons test *: p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001
To assay the reversal of CRISPRi repression of the mRFP test gene when IPTG inducer is removed, cultures of ES114:JMP1189/psgRNA (RR1) grown overnight with 2 mM IPTG induction of repression were re-cultured without IPTG inducer and assayed for restoration of RFP expression (Fig. 2B).
Functional testing of luxC repression
Bioluminescence is generated in V. fischeri when the autoinducer 3-oxo-C6 (Eberhard et al. 1981), activates the regulatory protein LuxR, which in turn activates transcription of the luxICDABEG operon. This operon contains luxI, (synthesis of the autoinducer 3-oxo-C6), luxAB (encoding dual-protein components luciferase enzyme), luxCDE (enzymes for synthesis of the luciferase aldehyde substrate) and luxG, a flavin reductase (Engebrecht et al. 1983; Engebrecht and Silverman 1984; Miyashiro and Ruby 2012; Nijvipakul et al. 2008). Since it has been shown that transcriptional repression caused by the steric hindrance of dcas9:sgRNA complex bound to a target gene is polar and blocks transcription of further downstream genes in an operon (Peters et al. 2016), we reasoned that targeting the luxC gene for repression would induce repression of the CDABEG genes of the operon and thus repression of bioluminescence.
The highest ranking prospective 20-nt targeting sequence abutting a 5’-NGG PAM sequence found near the initial sequence of the luxC gene (VF_A0923; Genbank: CP000021.2; 1440 bp) was identified by the CRISPy-web sgRNA design software (secondarymetabolites.org). Complementary BsaI tailed oligos (LC1 F/R; IDT; Table 1) matching the chosen luxC (LC1) target site were annealed and cloned into the triple BsaI cloning site of plasmid psgRNA (BsaI) via BsaI restriction/ligation generating plasmid psgRNA (LC1; Table 1). Plasmid psgRNA (LC1) was conjugated via tri-parental mating into V. fischeri strain ES114:pJMP1189. The time-course of inducible repression and subsequent de-repression of luminescence of cultured ES114:JMP1189 carrying plasmid psgRNA (LC1) is shown in Fig. 3.
Table 1.
Maximum CRISPRi Repression Levels
| Vector | Fold change under maximum repression |
|---|---|
| sgRNA(RR1) | ~ 11 |
| sgRNA(LC1) (in vitro) | ~ 20 |
| sgRNA(LC1) (in vivo) | ~ 29 |
| MMsgRNA(RR1) | ~ 8 |
| MMsgRNA(RR1,RR2) | ~ 14 |
| MMsgRNA(LC1) | ~ 14 |
| MMsgRNA(FA1) | ~ 20 |
Fig. 3.

Repression and subsequent derepression of luminescence. A repression of specific luminosity (relative light units/OD600) in OD600 = 2.0 cultures of uninduced control strain ES114:JMP1189/psgRNA(LC1) and IPTG (2 mM) induced ES114:JMP1189/psgRNA(LC1). B derepression of specific luminosity (relative light units/OD600) in OD600 = 2.0 subcultures of the same strains grown in media with no IPTG. Shown are median specific luminosities of three independent experiments (biological replicates). Error bars indicate the standard deviation. T-tests showed significant differences in means for the IPTG repressed ES114:JMP1189/psgRNA(LC1) and control while there was no significant difference between the derepressed ES114:JMP1189/psgRNA(LC1) and control. (*** indicates P < 0.001; ns indicates P > 0.05)
CRISPRi repression of bioluminescence in the light organ
The utility of ES114JMP1189/psgRNA (LC1) for regulating bioluminescence production from V. fischeri in its host environment during symbiosis was demonstrated by inoculating newly hatched aposymbiotic Euprymna scolopes juveniles with ES114JMP1189/psgRNA (LC1) cells that were induced by IPTG before and during the initial colonization of the juvenile squid. By manipulating the time-course of V. fischeri luminescence production during initial colonization, we can probe the time-dependence of the “winnowing” selection that prevents non-luminous symbionts from maintaining a successful colonization (Visick et al. 2000). Earlier research using non-luminescent luxA knockout mutants established that while mutant V. fischeri could successfully initiate colonization of the light organ, by 48 h post colonization the number of symbionts remaining in the light organ declined precipitously. This decline in light organ symbiont numbers then continued till no mutants were left, leading to an unsuccessful symbiosis (Visick et al. 2000).
In order to examine the ability to use IPTG-inducible CRISPRi gene repression of endogenous V. fischeri genes to modulate symbiont phenotypes during the course of symbiosis, we inoculated aposymbiotic hatchlings with ES114JMP1189/psgRNA (LC1) cells. Colonization was allowed to proceed for 24 h and subsequently IPTG was added to the hatchlings seawater to induce repression of luminescence. Luminescence levels from the hatchlings decreased by 48 h after infection and remained repressed out to 96 h, with a concomitant decline in light organ symbiont numbers (Figs. 4A, B), demonstrating the ability to successfully induce and maintain luminescence repression within colonized hosts.
Fig. 4.
A Luminescence of juvenile squid infected with multiplex ES114 strains. Luminescence of juvenile squid infected with different ES114:JMP1189/psgRNA(LC1) cultures grown with and without IPTG induction. Hatchlings were placed in seawater containing either (•) WT ES114 control, (▪) ES114:JMP1189/psgRNA(LC1) with no inducer, (▴) ES114:JMP1189/psgRNA(LC1) induced with 2 mM before infection, (▾) ES114:JMP1189/psgRNA(LC1) with no inducer before infection, but with 3 mM IPTG added to seawater at 24 h post infection. Mean luminescence values are averages for 5 animals for each treatment. All values reported are mean values and error bars reflect the standard deviation from the mean. Statistics were done using a two-way ANOVA, with Šídák’s multiple comparisons test *: p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. B CFU in colonized juvenile squid. CFUs per light organ of juvenile squid infected with different ES114:JMP1189/psgRNA(LC1) cultures grown with and without IPTG induction. E. scolopes hatchlings were exposed to seawater containing either (•) WT ES114 control, (▪) ES114:JMP1189/psgRNA(LC1) with no inducer, (▴) ES114:JMP1189/psgRNA(LC1) induced with 2 mM before infection, (▾) ES114:JMP1189/psgRNA(LC1) with no inducer before infection, but with 3 mM IPTG added to seawater at 24-h post infection. CFU/LO counts were obtained by sacrificing juvenile squids at serial 24-h time points post infection and plating the homogenate on SWT agar plates. CFU/LO values are averages for 5 animals for each treatment. All values reported are mean values and error bars reflect the standard deviation from the mean. Statistics were done using a two-way ANOVA, with Šídák’s multiple comparisons test *: p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001
Multiplexing sgRNA plasmid vectors
As a final component of a generalized CRISPRi tool suite for V. fischeri, we investigated designs to combine the expression of multiple sgRNA transcripts from our psgRNA vector, which would allow for simultaneous repression of multiples genes-of-interest, or to target a single genetic locus with multiple sgRNA transcripts. Targeting multiple non-overlapping sgRNAs to a single gene can additively increase CRISPRi repression of transcription of that gene (Qi et al. 2013). We estimated that having two nearly homologous sgRNA cassettes in the same plasmid would lead to recombinational instability in V. fischeri (which has a generally high level of recombinational functionality (Thompson et al. 2007)) and therefore adopted a design that utilized nonhomologous sgRNA cassette component sequences that can be cloned into a single expression plasmid with minimal risk of recombination (Reis et al. 2019). A software sgRNA design tool (ELSA Calculator; De Novo DNA) was used to find combinations of non-homologous sgRNA cassette components (synthetic minimal promoters, RBS, sgRNA frameworks, transcription terminators) to enable the design of a sequence with three independent sgRNA cassettes that was cloned into our ES114:JMP1189 single sgRNA plasmid, replacing the sgRNA/lacI (BsaI) cassette. This generated the multimeric sgRNA cloning plasmid pMMsgRNA (3IIS). The three Type IIS spacer cloning sites can be sequentially restriction digested and cloned with tailed annealed oligomers, using the same protocol used for cloning spacer elements into the single psgRNA (BsaI) cloning plasmid. We generated multiple multiplexed sgRNA plasmids with combinations of 20-nt spacer targeting sequences and validated their function and utility in vitro. Two of the targeting spacers combined in multiplex format were the previously validated spacers for mRFP (RR1) and luxC (LC1), along with a spacer targeting the master regulator of the flagella production regulon, flrA (FA1) (Millikan and Ruby 2003; Wolfe et al. 2004). To test for enhancement of single gene repression through multiplexed 20-nt spacer targeting, we compared the repression of the test mRFP reporter in ES114:JMP1189 using the single 20-nt pMMsgRNA (RR1) with a version expressing two sgRNA to mRFP (pMMsgRNA (RR1, RR2). A triple sgRNA multiplex with targeting spacers for mRFP, luxC, and flrA was also able to cause significant fluorescence repression (Fig. 5).
Fig. 5.

Multiplexed sgRNA repression of mRFP fluorescence. A Cultures grown with no IPTG; B Cultures grown with 2 mM IPTG induction of repression to an OD600 of 0.4, with triplicate 200 mL aliquots assayed for fluorescence in a 96-well multimodal plate-reader. Specific fluorescence (relative fluorescence units/OD600; excitation 584 nm, emission 607 nm) of the single and multiplexed sgRNA plasmid carrying strains was normalized to the control strain ES114:JMP1189 (mRFP + , no sgRNA). Columns indicate mean values of three independent experiments (biological replicates). Different letters on the abscissa denotate significant differences between groups according to the Tukey post-hoc comparison
This triple multiplex sgRNA plasmid was also assayed for its ability to repress luminescence and bacterial motility. Figure 6 shows titratable repression of luminescence in ES114:JMP1189/pMMsgRNA (RR1, LC1, FA1) cells in culture supplemented with 0–2 mM IPTG. Functional testing of CRISPRi repression of the third targeted gene in the multiplex sgRNA plasmid, flrA, revealed severe repression of cell motility in a standard soft-agar motility assay (Fig. 7).
Fig. 6.

Multiplexed sgRNA repression of luminescence. ES114:JMP1189/pMM(RR1, LC1, FA1) cells were sub-cultured in SWT with the indicated levels of IPTG inducer and grown to an OD600 of 2.0, with triplicate 200 mL aliquots assayed for luminescence in a 96-well multimodal plate-reader. The specific luminescence (relative luminescence units/OD600) of the single and multiplexed sgRNA plasmid carrying strains was normalized to the control strain ES114. Columns reflect mean values; error bars indicate standard deviations. Different letters on the abscissa denotate significant differences between groups according to the Tukey post-hoc comparison
Fig. 7.
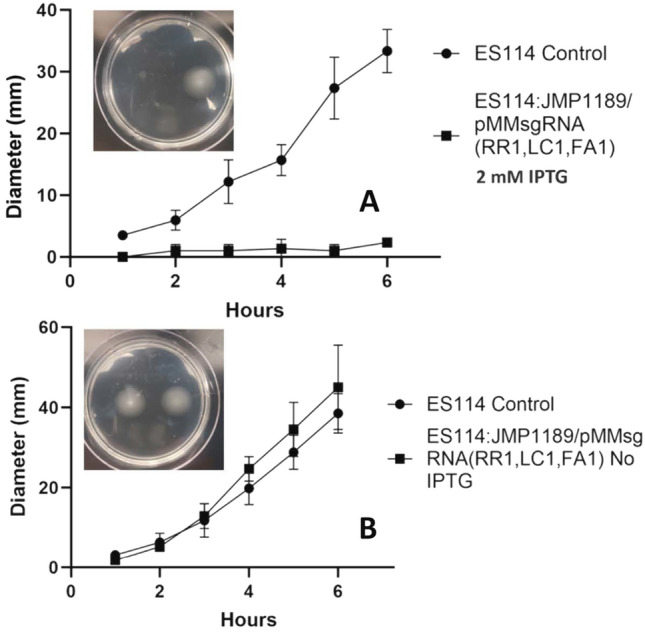
Motility of ES114:JJMP1189/pMMsgRNA(RR1,LC1,FA1). A Migration of ES114:JJMP1189/pMMsgRNA(RR1,LC1,FA1) sub-cultured with 2 mM IPTG and spotted on TBS-Mg2+ agar with 2 mM IPTG. B Migration of ES114:JJMP1189/pMMsgRNA(RR1,LC1,FA1) sub-cultured without IPTG and spotted on TBS-Mg2+ agar. Inserts show representative motility plates measured at 6 h. Statistics were done using a two-way ANOVA, with Šídák’s multiple comparisons test *: p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.Error bars reflect standard deviations. *: p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001
Discussion
dCas9-based CRISPRi inhibits transcription in bacterial systems during mRNA transcription initiation or elongation through steric hindrance, physically blocking RNA polymerase progression (Banta et al. 2020a, b). The targeting sequence requirements for the popular S. pyogenes dcas9 (Spdcas9)—i.e., a 20-nt target sequence next to an upstream 5’-NGG PAM sequence (Jinek et al. 2012) suggests that the vast majority of bacterial genes (or other loci) will be accessible to dcas9/sgRNA binding. The V. fischeri CRISPRi system of vectors developed in this work is a relatively streamlined general purpose tool for programmable control of either single or multiple gene(s) expression which is highly flexible and modifiable for diverse applications. The system can efficiently repress both exogenous (mRFP) and endogenous (luxC, flrA) genes in V. fischeri, both when grown in culture and within their symbiotic host.
Our V. fischeri CRISPRi system uses stable plasmid vectors (no antibiotic selection pressure required to maintain the plasmid; Dunn et al. 2006) for expression of programable sgRNA transcripts in conjunction with an attTn7 site integrated dcas9 expression cassette. The Tn7 transposases recognize a site in the highly conserved glmS gene (essential in E. coli (Milewski 2002) and likely essential in nearly all bacteria), and inserts immediately downstream of glmS into the attTn7 site, such that no gene is disrupted with no discernable fitness cost to the host (Peters and Craig 2001). Transposition occurs exclusively into attTn7, and not into other locations (Peters and Craig 2001), and allows for the stable integration of genes in single copy. Transgene expression from the attTn7 site has not been seen to have, nor been affected by, any polar effects with neighboring loci (Chang et al. 2024).
Design and construction of sgRNA plasmids targeting any gene/locus-of-interest is a simple process of Type IIS restriction cloning of 20-nt spacer sequences (as annealed pairs of oligonucleotides) into the psgRNA (BsaI) cloning plasmid that are complementary to the non-template strand of the target gene/locus and adjacent to the required PAM (5’-NGG; Fig. 1). Software packages have been developed and are available on-line for identifying such target sequences in sequenced genomes (Blin et al. 2016; www.synthego.com; https://crispy.secondarymetabolites.org). Target sequences can also be found by manually searching of the gene(s)-of-interest sequence, but the sgRNA software performs additional laborious quality checks such as searching the V. fischeri genome for off-target sequences that have unintended full or partial matches to any putative 20-nt targeting sequences (Blin et al. 2016, 2020).
Initial function validation of our CRISPRi system used a sgRNA plasmid targeting a chromosomally integrated mRFP gene for repression. IPTG inducer supplementation of the media of exponentially growing mRFP tagged ES114 cells (ES114:JMP1189) carrying the psgRNA (RR1) plasmid showed titratable repression of mRFP reporter fluorescence at IPTG concentrations in a range of 0.05 to 2.0 mM/mL (Fig. 2). We observed significantly greater repression from this plasmid-based system, compared to the fully integrated CRISPRi strain ES114:JMP1183. This is consistent with reports that sgRNA transcript levels can be limiting in CRISPRi applications (Fontana et al. 2018), (Peters et al. 2019; Byun et al. 2023); our multicopy plasmid (~ 10 copies/cell) sgRNA expression provides a higher molar ratio of sgRNA transcripts to dcas9 enzymes in the cells, which would facilitate higher rates of binding and inducing steric repression (Banta et al. 2020a, b). To ensure that there was a saturating amount of lacI repressor protein present in the cells to bind to the multiple PLlac0-1 promoters on the psgRNA plasmids, a lacI expression cassette was included on each sgRNA expression plasmid, in addition to the lacI expression cassette included in the attTn7 integrated dcas9 cassette.
CRISPRi modulation of symbiont gene expression and phenotype during host colonization
Work in genetic tool development for marine bacteria is continually expanding, reflecting their wide-spread associations in aquatic microbiomes. α-proteobacteria and γ-proteobacteria symbionts have been subject to genetic modification for research in phytoplankton (Sunagawa et al. 2015), coral (Bourne et al. 2016), tubeworm (Vijayan et al. 2019), and sepiolid squid (Dunn et al. 2006; Visick et al. 2018) symbiotic systems. Recently, a novel CRISPRi plasmid tool was used to demonstrate the ability to modulate gene expression in the symbiont marine bacterium Pseudoalteromonas luteoviolacea during the course of its symbiosis with the model tubeworm, Hydroides elegans (Alker et al. 2023). In their study, CRISPRi repression of the macB gene, which is essential for inducing host tubeworm metamorphosis, was shown to lead to significantly reduced levels of metamorphosis in the host.
To demonstrate the utility of our CRISPRi system for modulating endogenous V. fischeri gene expression in symbionts within the host squid, we chose to target bioluminescence production during squid hatchling colonization. The production of bioluminescence by V. fischeri during the initial colonization of host tissues, and latter during the maintenance of the symbiosis within the mature light organ, is intricately coordinated by multiple environmental conditions and cues within host microenvironments (Pan et al. 2015; Chavez-Dozal et al. 2012; Nourabadi and Nishiguchi 2021), to ensure that bioluminescence is rhythmically produced in a diurnal pattern (Schwartzman et al. 2015). Bioluminescence produced by V. fischeri has also been found to act as a cue for changes in the tissue development of the immature colonized light organ (Visick et al. 2000), with hosts colonized by non-luminescing mutant V. fischeri strains failing to undergo normal light organ epithelial tissue maturation. These “dark” mutants are also actively rejected by the host after initial colonization, implying that the regulation of bioluminescence by microenvironmental conditions/cues (pH, oxygen saturation, nutrient status, cell density/quorum signaling) found within the host is also accompanied by an ability of the host to monitor the proper bioluminescence response from the symbionts (Visick et al. 2000; Chavez-Dozal et al. 2012; Nourabadi and Nishiguchi 2021; Pipes and Nishiguchi 2022). Inducible CRISPRi repression of bioluminescence provides an experimental tool for over-riding the normal regulation of bioluminescence during colonization; in our proof-of-concept application we were able to repress luminescence 48 h after initial colonization of hatchlings and recapitulate the decline in symbionts seen during initial colonization with mutant dark symbionts (Visick et al. 2000). Currently we are unable to extend the time course of our experiments past 96 h as we have been unsuccessful in raising the juvenile squid past this point. If this husbandry hurdle can be crossed, future studies that follow our CRISPRi repressible luminescence strains past our current 96 h time-course will be able to reveal the effects of reversing the repression and restoring luminescence in the remaining symbionts.
Bioluminescence in V. fischeri is produced by the luciferase enzyme, a heterodimer of LuxA and LuxB proteins. luxA and luxB are members of the lux operon (luxCDABEG). The LuxR-AHL transcriptional activator complex binds to the promoter region of the operon and activates polycistronic transcription of the genes.
CRISPRi repression of any of the genes in the lux operon would lead to loss of bioluminescence resembling the mutations (natural and artificial) in different lux operon genes that result in “dark” mutant strains (O’Grady and Wimpee 2008). We chose to target the luxC locus because it is the first gene in the operon and steric hindrance at the luxC locus would produce a polar repression (Peters et al. 2016) of the remaining genes in the operon (luxDABEG). Functional validation of in vitro bioluminescence repression was conducted using strain ES114:JMP1189 carrying the psgRNA (LC1) plasmid. The maximum level of repression was different than that found in validation tests of the mRFP targeting plasmid psgRNA (RR1)—this is consistent with work showing the level of repression achieved using CRISPRi being gene-specific (Banta et al. 2020a, b), and the time course required for loss of function through transcriptional repression reflecting the stability (half-life) of the protein(s) being repressed, as well as the growth rate of the cells (lowering functional levels of the repressed protein through dilution during cell division) (Qi et al. 2013). The times observed for maximally induced repression, and the lifting of that repression to initial levels in the mRFP test strain could serve to guide gene function experiments completed in similar cell culture conditions but are not as useful to guide research using timed CRISPRi repression of genes during V. fischeri host colonization. The growth pattern and phenotype of cells in an established light organ colonization are notably changed compared to the growth pattern, and phenotype, of the same cells grown in culture (Chavez-Dozal et al. 2014; O’Shea et al. 2005). During the rhythmic diel cycle of the light organ, 95% of the symbionts in the light organ are expelled at dawn. The remaining cells repopulate the light organ crypt microenvironments through rapid growth, reaching maximal cell density levels by noon (Norsworthy and Visick 2013). The rate of growth then diminishes abruptly (generation time increasing from ~ 0.5 h to 10–18 h) and cells can be observed to become reduced in size and non-flagellated (Ruby and Asato 1993). Since accurate estimations of the rate of timing of repression and de-repression of the luxC gene in the light organ microenvironment could not be generated in culture, the timing of induction of CRISPRi repression in the colonized squid experiments had to be determined empirically.
ES114:JMP1189 carrying psgRNA (LC1) was used to inoculate abiotic hatchling squid to demonstrate the ability of inducible timed repression of bioluminescence in vivo to probe the temporal monitoring of symbiont bioluminescence by the host (Fig. 5). The addition of IPTG to the hatchlings seawater at 24 h post inoculation was able to induce and maintain repression of bioluminescence from the symbionts to levels that triggered the same pattern of loss of symbiont numbers seen during early stages of colonization with dark lux mutants (Koch et al. 2014). Notably, the drop in CFU/LO at 55 h post inoculation followed the same pattern as seen at 24 h in “dark” ES114:JMP1189 carrying psgRNA (LC1) that were fully IPTG induced at inoculation. Our results demonstrate the utility of inducible CRISPRi repression in probing the temporal distribution of regulatory conditions (bioluminescence) and responses (symbiont rejection) during the symbiosis.
Multiplex plasmid design
CRISPRi systems that target single genes enable relatively rapid and facile transcriptional repression for forward genetics studies, avoiding laborious mutant strain construction methodologies that result in altering the native regulation of the gene (Qi et al. 2013; Peters et al. 2019; Mimee et al. 2015; Rock et al. 2017; Jiang et al. 2024). Simultaneous CRISPRi targeting of multiple genes in the same cell (multiplex CRISPRi) (Larson et al. 2013; Qi et al. 2013; Peters et al. 2016; Zhao et al. 2016) avoids the even more laborious construction of multiple-deletion strains. Multiplex CRISPRi systems have been demonstrated in various bacterial species using a variety of methods to express multiple sgRNAs—including crRNA arrays (Kim et al. 2017), multiple arrayed independent sgRNA cassettes (Qi et al. 2013; Peters et al. 2016), and nonrepetitive extra-long sgRNA arrays (ELSAs; Reis et al. 2019).
Our novel V. fischeri multiplex sgRNA plasmid uses an ELSA cassette for the simultaneous expression of three sgRNAs. Each sgRNA cassette within the multiplex plasmid had a unique strong synthetic constitutive promoter, a 20-nt spacer sequence targeting an independent genetic locus, functional sequence variant sgRNA framework sequences, and unique transcription terminators and spacing sequences. With this design, no sequence part in the three sgRNA cassettes shares more than 17 bp homology with another part. This lack of homology, compared to the > 90% sequence homology between repeated sgRNA cassettes has two advantages: (1) plasmids with repeated sequences (areas of homologous sequence) can recombine in highly recombinogenic bacteria like V. fischeri (Lin et al. 2018), altering the content of the plasmid or rendering it unstable for replication, and (2) Synthetic dsDNA construct services cannot synthesize sequences containing areas with homologous repeats (Reis et al. 2019). Notably, the three unique sgRNA cassettes could not use the same PLlac0-1 inducible promoter used in the single sgRNA cassette plasmids (excessive sequence homology) and having three different inducible promoters would make it logistically difficult to utilize. Inducibility of the multiplexed CRISPRi system comes from the single PLlac0-1 promoter driving the integrated dcas9 cassette in the parent ESS114:JMP1189 strain.
We observed higher mRFP repression from the multiplex plasmid with two non-overlapping mRFP spacer sequences compared to the multiplex plasmid with a single mRFP spacer sequence, demonstrating the ability of a multiplexed sgRNA plasmid to generate increased target gene repression by providing two simultaneous sites of steric hindrance (Qi et al. 2013). The ability of a multiplex sgRNA plasmid to simultaneously repress three separate genes was demonstrated using assays for mRFP fluorescence, bioluminescence, and motility on soft-agar plates. The flrA regulatory gene was chosen as a gene-of-interest in this study because it has also been shown in prior research (Chavez-Dozal et al. 2012; Millikan and Ruby 2003; Shrestha et al. 2022) to be the master transcription activator of the flagella production regulon (which contains other non-flagella associated operons; Millikan and Ruby 2003). In V. fischeri, flagellar gene regulation is controlled in a multilevel cascade (Norsworthy and Visick 2013), with FlrA activating early flagellar genes, including flrBC and fliA. Expression of the late genes, multiple flagellar filament and motor proteins, is regulated in a sequential cascade first by FlrBC and then FliA (Aschtgen et al. 2019). flrA is necessary for initial colonization of the light organ, but its expression is repressed via unknown signals and cues in the light organ where most symbionts appear non-flagellated (Ruby and Asato 1993), only to be re-expressed shortly before symbionts are expelled at dawn (Norsworthy and Visick 2013). When insertional (DM126, DM127, and DM128) and partial deletion (DM159) mutations of the flrA gene were constructed they were shown to be non-motile in soft agar motility plates (Millikan and Ruby 2003). Additional ΔflrA knockout mutants in V. fischeri, generated by targeted homologous recombination, were also found to be non-motile in soft-agar (Visick et al. 2018).
The work presented demonstrates proof-of-principle utility of a CRISPR interference suite of vectors and strains for inducible repression of expression of both exogenous and endogenous genes-of interest via simple targeting with target sequence complementary 20-nt spacer sequences cloned into sgRNA expression plasmid vectors. The single IPTG inducible sgRNA expression plasmids, used with a common genomically integrated IPTG inducible dcas9 ES114 strain, can repress V. fischeri gene expression in a titratable and reversable course in culture, as well as in symbionts colonizing juvenile host squid. A multiplex version of the sgRNA plasmid, though not inducible itself, provides the capability for elevated repression of a single gene via multiple independent complementary targeting spacer sequences to that gene, or can simultaneously target up to three genes for repression. CRISPR interference is a powerful, flexible general genetic programming tool for research in the V. fischeri—E. scolopes symbiosis model system, complementing and extending the current toolbox of gene modification methodologies and allowing for determining the exact timing of the genetic cross talk between hosts and their beneficial microbes.
Materials and methods
Strains and media
Strains and plasmids used in this study are listed in Table S1. Oligos, and synthetic dsDNA constructs used in this study are listed in Table S2. Escherichia coli strains DH5α and pir + competent GT115 (InvivoGen) were used for plasmid maintenance, cloning, and conjugation. V. fischeri strain ES114 was grown aerobically using various media at 28 °C. For molecular biology applications and fluorescence assays, V. fischeri cells were cultured in Luria–Bertani high salt (LBS) containing 10 g of tryptone, 5 g of yeast extract, 20 g of NaCl, and 20 mM Tris-hydrochloride (Tris–HCl, pH 7.5) per L of sterile water. All squid colonization and bioluminescence assays used seawater tryptone (SWT), which contained 5 g of tryptone, 3 g of yeast extract, and 700 mL of artificial seawater (ASW) per L of sterile water. E. coli, aerobically grown at 37 °C, was cultured in Luria–Bertani (LB) media, which contained 10 g tryptone, 5 g yeast extract, and 10 g NaCl. Agar was added to the media at 15 mg/mL for plating. Where appropriate, the antibiotics chloramphenicol and kanamycin were added to growth media at 1–3 and 100 μg/mL, respectively, for V. fischeri with ampicillin, chloramphenicol and kanamycin added to growth media at 100, 30, and 100 μg/mL respectively, for E. coli. Thymidine (0.3 mM) was used to supplement LB media for culturing of the thy-auxotroph DH5α strain carrying conjugation helper plasmid pEVS104. IPTG (Isopropyl ß-D-1-thiogalactopyranoside) (GoldBio), for CRISPRi induction was added to media when appropriate as indicated.
Artificial seawater for use with E. scolopes hatchlings was made using ddi H20 (1L) with 30 g Instant Ocean® marine salt (Instant Ocean Spectrum Brands) and 10 g Marine Mix® marine salt (Bulk Reef Supply). Artificial saltwater was aerated with aquarium air-pumps and air-stones.
Molecular cloning
All molecular cloning reagents (restriction enzymes) are from NEB. Plasmid extraction Plasmid Miniprep Kits (ZymoPURE™) from Zymogen. Primers were ordered from IDT DNA. Synthetic dsDNA constructs were ordered from Twist Biosciences.
Design of a V. fischeri CRISPRi suite of vectors
Our experimental goals were to create an inducible CRISPRi system able to target and repress single or multiple genetic loci in V. fischeri, both in culture and in symbiosis with their squid host. These goals lead to several design constraints-
The dcas9 and sgRNA components must be stably retained through prolonged growth periods—up to several days of growth in infected squid. The use of antibiotic selection to enforce retention of plasmid vectors is problematic because of potential toxicity of common antibiotic selective agents with the squid host, and incomplete penetration of antibiotics within the light organ crypts (Dunn et al. 2006).
The inducible promoter(s) used to control expression of CRISPRi components should have minimal leaky expression when uninduced and the inducer used with the system, when added to sea-water, must be non-toxic to the squid host, and be able to penetrate the crypts of the light organ.
Express sgRNA in excess of dcas9 to ensure that the proportionately lower concentration of individual sgRNA transcripts produced from a multiplex (three sgRNA cassette) vector can saturate the expressed dcas9.
Successful CRISPRi systems have been developed that genomically integrate both dcas9 and sgRNA (Liu et al. 2017; Peters et al. 2016), as well as systems that contain dcas9 and sgRNA on dual or single stable plasmid vectors (Rachwalski et al. 2024; Tan et al. 2018). For our goals though, heterologous systems, with an integrated dcas9 and plasmid expressed sgRNA (Rousset et al. 2018) fit all of the design constraints.
dcas9 cassettes have been previously integrated into the V. fischeri genome, at the intergenic attTn7 Tn7 transposon site, via conjugation of an integrating plasmid vector(Geyman et al. 2024). Tn7 integrated dcas9 cassettes have been shown to be stably retained through > 50 generations of antibiotic selection-free growth in culture while maintaining dcas9 expression (Peters et al. 2019). Stable V. fischeri plasmids have been developed, derived using the native pES213 plasmid origin of replication, which are stable (Dunn et al. 2005) during colonization of the squid. pVSV105 is a widely utilized conjugatable pES213 derived plasmid (copy number of 9.4 ± 2.4) which has been found to be ≥ 99% retained in V. fischeri after nonselective growth in culture for 60 generations, or 72 h of squid colonization (Dunn et al. 2006).
IPTG inducible promoters, such as the Plac, Pma, and PLlac0-1 promoters, have been successfully used in V. fischeri, both in culture and in squid hosts (Geyman et al. 2024; Visick et al. 2018). IPTG is non-toxic to the squid host and penetrates the light organ crypts (Visick et al. 2018). Dual PLlac0-1 promoters controlling both dcas9 and sgRNA expression were recently used in the attTn7 integrating Mobile-CRISPRi system to repress the rpoB essential gene in V. fischeri. The system exhibited negligible non-induced dcas9 expression, while showing significant induced rpoB repression (Geyman et al. 2024).
The multicopy stable pVSV105 plasmid provides ~ 10 sgRNA expression cassettes for every single genomic dcas9 expression cassette. Using the same PLlac0-1 inducible promoter for both provides coordinated induction of both components, with an excess of sgRNA. This excess of sgRNA transcripts, due to using the same promoters for dcas9 and sgRNA while having the dcas9 integrated and the sgRNA expressed from multiple plasmids, is an elegant way to ensure that in the multiplex version of the sgRNA plasmid there is enough of each individual sgRNA transcript to saturate the expressed dcas9 (Luo et al. 2015; Reis et al. 2019; Vigouroux and Bikard 2020). In a CRISPRi study in Zymomonas mobilis using independently inducible promoters for dcas9 and sgRNA expression, it was noted that the concentration of sgRNA relative to the concentration of dcas9 was a limiting factor in CRISPRi repression (Banta et al. 2020a, b).
Tn7 transposon integrated dcas9 expression V. fischeri strain construction.
A Mobile-CRISPRi Tn7 transformation donor plasmid developed for conjugational transfer into γ-proteobacteria (pJMP1183-Addgene, #119254; (Peters et al. 2019)) was used to transfer a mRFP (monomeric red fluorescent protein) expression cassette (for use as a repression target in testing), the lacI gene needed for IPTG induction, an IPTG inducible (PLlacO-1 promoter) dcas9 cassette, an IPTG inducible (PLlacO-1 promoter) sgRNA (RR1) expression cassette targeting the introduced mRFP gene for repression, and a KanR selection marker into V. fischeri ES114 at the attTn7 locus to generate strain ES114:pJMP1183. We also separately transferred pJMP1189 (Addgene, #119257; Peters et al. 2019) to introduce an IPTG inducible (PLlacO-1 promoter) dcas9 cassette, the lacI gene needed for IPTG induction, a KanR selection marker, and an mRFP expression cassette (for use as a repression target in testing, and later as a fluorescent cell tag) into V. fischeri ES114 at the attTn7 locus to generate strain ES114:pJMP1189. These were transferred to V. fischeri ES114 by tetra-parental conjugation with the helper E. coli strains CC118 λpir + /pEVS104 thy- (pESV104 contains conjugative transfer genes and is a thymidine auxotroph) and BW25141/pJMP1039 (expresses Tn7 transposase; see Table S1). (Christensen et al. 2020). Briefly, E. coli donor strains (carrying Tn7 transposon plasmids pJMP1183, and pJMP1189, respectively) and E. coli helper strain Dh5a thy- carrying pEVS104 (Eric Stabb and Ruby 2002) along with a second E. coli helper strain carrying transposase (pJMP1039, Addgene # 119239; Peters et al. 2019) were cultured individually overnight in 5 mL LB media at 37 °C with appropriate antibiotics and additives. V. fischeri strain ES114 was cultured overnight (14 h) in SWT media at 28 °C with shaking. All cultures were then sub-cultured (100 μL aliquots into 5 mL appropriate media) and grown at their respective temperatures with shaking for 3–4 h. 250 μL of each of the four cultures were combined into a 1.5 mL microcentrifuge tube (Eppendorf). A 250 μL donor only control from each of the three E. coli cultures was placed in 1.5 mL microcentrifuge tubes, and a 250 μL recipient-only (ES114) control was placed in a 1.5 mL microcentrifuge tube. Cells were then pelleted at 8000 rpm for 5 min in a microcentrifuge (Eppendorf), and the supernatant discarded leaving 10–20 μL liquid in the tube. The pellets were resuspended in this liquid, and 100 μL of each tube was spotted in the middle of a pre-warmed LBS agar plate. After the liquid had absorbed into the plates, they were inverted and incubated overnight in a 28 °C incubator. In the morning, the colony was scraped off with a sterile pipette tip and the cells resuspended in 1 mL LBS by vortexing briefly. 100 μL aliquots of each cell culture were spread onto antibiotic containing LBS agar plates for selection of attTn7-site integrated transformed colonies (in the case of pJMP1183 and pJMP1189 this is Kanamycin, 100 μg/μL). Plates were incubated at 18 °C to repress growth of donor E. coli, and colonies of transformed V. fischeri selected when they appeared after 1–3 days. 20 of these colonies were isolated onto master LBS Kan 100 μg/mL agar plates and tested for the presence of integrated CRISPRi transposon construct by patching the master plate colonies on LBS with ampicillin agar and LBS kanamycin agar and incubating the plates at 28 °C overnight. Strains that have integrated the transposon segment and no longer retain the donor plasmid are AmpR-, KanR+. Further confirmation of genomic integration at the attTn7 site is completed colony PCR using primers flanking the attTn7 insertion site.
sgRNA expression plasmids construction
Single sgRNA cassette expression plasmids
Single sgRNA expression plasmids were made using the V. fischeri stable shuttle vector pVSV105 as a template for PCR amplification of a vector backbone segment containing the Vf oriV, an incP oriT, the R6Kg oriV, and a CamR cassette, using tailed primers to provide ~ 30 bp homologous end overlap to the insert to be cloned. For the single (no targeting spacer sequence) sgRNA plasmid psgRNA, the empty sgRNA (triple-BsaI) expression cassette from plasmid pJMP1339 (Addgene #119271) was PCR amplified with tail primers that provided ~ 30 bp homologous end overlap to the pVSV105 backbone PCR amplicon (See Table S2 for sequences). These pieces were cloned into pir + GT115 competent E. coli using the NEBuilder® HiFi DNA Assembly Cloning Kit (NEB; Cat. # E5520S), following NEB protocol. Clones were selected as colonies on chloramphenicol antibiotic plates and the presence of correct constructs was confirmed by colony PCR with primers spanning cloning joints (Table S2).
Constructing 20-nt targeting sequences
The highest ranking candidate sgRNA spacer 20-nt sequences targeting the mRFP1, mRFP2, luxC, and flrA genes were found with the CRISPy-web on-line sgRNA software (CRISPy-web; secondarymetabolites.org), using the V. fischeri ES114 reference genome (GenBank: GCA_000011805.1) to identify optimal dcas9 targeting sites that have no potential off-target sites (sequences with more than 9/20 homologous bases to the spacer sequence). Candidate spacers closest to the 5’ end of the ORFs were always chosen since studies of the efficacy of sgRNA targeting locations along several gene sequences suggest that achieving high efficacy gene knockdown in Vibrio species necessitates targeting toward the 5′ end of the open reading frame (Geyman et al. 2024). Additional sequence was added to the 5’ end of these sequences to provide compatible single strand overhangs that match the restriction sites of the sgRNA plasmid spacer cloning site (BsaI for the single sgRNA plasmids, and BbsI, BsaI, and BaeI for the multiplex plasmid spacer cloning sites. Complementary pairs of annealed 5’-phosphorylated oligos with these 20-nt spacer + single strand overhangs were ordered from IDT DNA. See Table S2 for sequences of oligos.
Cloning 20-nt targeting sequences into empty sgRNA (triple-BsaI) plasmids
The annealed oligo spacer sequences targeting the mRFP, luxC, and flrA genes were cloned into the triple-BsaI targeting sites of psgRNA plasmids via ligation of annealed oligos (Brooks et al. 2014). 2 μL of a 1:20 dilution of the annealed oligos was ligated to 100 ng BsaI-digested psgRNA for 30 min at room temperature following the manufacturers protocol (NEB.T4 Ligase, M0202). The ligation mixture was used to transform ChemiComp GT115 Competent E. coli (Invivogen, Cat. Code gt115-11, 0.1 mL aliquots) following the manufacturers protocol. Briefly, 100 μL of frozen GT115 cells were thawed on ice and then transferred into a chilled 1.5 mL tube. 5 μl of the ligation reaction was added to the cells, mixed, and returned to ice for 30 min. The cells were heat shocked in a 42 °C water bath for 30 s and then returned to ice for two minutes. 900 μL of room temperature SOC medium was added to the reaction and the cells were incubated for one hour at 37 °C with shaking (250 rpm). 200 μL of the reaction was spread on LB agar plates with 50 μg/mL chloramphenicol and incubated overnight at 37 °C. 20 isolated colonies were collected the next day and spotted with a sterile pipette tip on a master plate (LB agar plates with 50 ug/mL chloramphenicol) which was grown overnight at 37 °C. When spotting the master plate, the pipette tip was also used to transfer some of the colony to a 50 μL PCR reaction mix in a 0.2 mL PCR tube. Colony PCR was performed using Q5 Hi-Fi 2X MasterMix (NEB, Cat. # M0492S) per the manufacturers protocol. The thermocycling conditions for the reaction were: Initial denaturization 98 °C 30 s, 25 cycles @ 98*C denaturation 10 s, annealing 66–70 °C (primer dependent, see Table S2 for primer sequences) 30 s, extension 72*C 30 s. A final extension at 72*C 2 min, and then held at 4 °C. 10 μL of each colony PCR reaction was mixed with 2 μL 6X Purple Loading Dye (NEB; Cat. #B7024S) and run on a 1% agarose gel in TAE buffer at 75 V until the loading dye front was ~ ¾ down the gel. 1 μL of 1 kb Plus DNA Ladder (NEB; Cat. #N3200S) mixed with 1 μL 6X Purple Loading Dye (NEB; Cat. B7024S) and 4 μL TAE buffer was added to the gel as a size standard.
Multiplex sgRNA cassette expression plasmids
The multiplex plasmid pMMsgRNA (3TIIS) expression cassette, with an array of three separate sgRNA expression cassettes, was designed using the Extra Long sgRNA Array (ELSA) software (De Nova DNA; https://salislab.net/software/design_elsa_calculator) to contain three programable sgRNA cassettes (BbsI, BsaI, and BaeI spacer cloning sites) with non-homologous sequences. The insert was ordered as a synthetic dsDNA construct with 30 bp homologous ends (TWIST Bioscience, CA, USA) and cloned into a PCR amplified (primers pVSV105 cassette/MMsgRNA F/R (Table S2)) pVSV105 backbone using the NEBuilder® HiFi DNA Assembly Cloning Kit (NEB Cat. #E5520S) following the NEB protocol. The resulting plasmid, pMMsgRNA(3TIIS), is an empty cloning vector which can express three independent targeted sgRNAs from three different constitutive promoters. Cloning a 20-nt targeting spacer into a multiplexed sgRNA plasmid follows the same protocol described above for designing and cloning the spacer, and follows the same protocol for transforming the targeted multiplex vector into a V. fischeri. Strain with integrated dcas9. While the three sgRNA cassettes are not IPTG inducible, the dcas9 is—so the multiplex CRISPRi system is inducible, but not independent. Four additional multiplex plasmid were constructed using a different protocol (—for these plasmids the synthetic dsDNA construct was designed to already contain 20-nt spacer sequences in the three sgRNA cassettes. All that was needed to construct these plasmids was to use HiFi Assembly (see above) and transform into V. fischeri (see below).
pMMsgRNA(RR1)—targets site 1 in mRFP.
pMMsgRNA(RR1:RR2)—targets sites 1 & 2 in mRFP.
pMMsgRNA(RR1:LC1)—targets site 1 in mRFP and site 1 in luxC.
pMMsgRNA(RR1:LC1:FA1)—targets site 1 in mRFP, site 1 in luxC, and site 1 in flrA.
Dcas9 and dcas9/sgRNA plasmid V. fischeri strain construction.
Complete inducible CRISPRi V. fischeri strains were generated by introducing an inducible sgRNA expression plasmid, such as psgRNA (this study), from an E. coli cloning host by triparental conjugation with an E.coli thymidine- auxotroph containing conjugative helper plasmid pEVS104 (Stabb and Ruby 2002) and the appropriate V. fischeri recipient (ES114:pJMP1183 and ES114:pJMP1189) as described previously (DeLoney et al. 2002). Briefly, E. coli donor strains (i.e. psgRNA) and E. coli helper strain DH5α thy- carrying pEVS104 (Stabb and Ruby 2002) were cultured individually overnight in 5 mL LB media at 37 °C with appropriate antibiotics and additives. A recipient V. fischeri strain (e.g. ES114:pJMP1183; mFRP + , KanR) was cultured overnight (14 h) in SWT media at 28 °C with shaking. All cultures were then sub-cultured (100 μL aliquots into 5 mL appropriate media) and grown at their respective temperatures with shaking for 3–4 h. 250 μL of each of the three cultures were combined into a 1.5 mL microcentrifuge tube (Eppendorf). A 250 μL donor only control from each of the two E. coli cultures was placed in 1.5 mL microcentrifuge tubes, and a 250 μL recipient-only control was placed in a 1.5 mL microcentrifuge tube. The cells were pelleted at 8,000 rpm for 5 min in a microcentrifuge (Eppendorf), and the supernatant discarded leaving 10–20 μL liquid in the tube. The pellets were resuspended in this liquid, and 100 μL of each tube was spotted in the middle of a pre-warmed LBS agar plate. After the liquid had absorbed into the plates, they were inverted and incubated overnight in a 28 °C incubator. In the morning, the colony was scraped off with a sterile pipette tip and the cells resuspended in 1 mL LBS by vortexing briefly. 100 μL aliquots of each cell culture were spread onto LBS agar plates containing the appropriate antibiotic to select for plasmid transformed colonies (Chloramphenicol, 1–3 μg/μL). The plates are incubated at 18 °C to repress growth of donor E. coli, and colonies of plasmid bearing V. fischeri can be selected when they appear after 1–3 days. 20 of these colonies are isolated onto master LBS Kan 100 μg/mL agar plates and tested for the presence of the plasmid using plasmid extraction kits (Zymogen) and restriction digest analysis. Strains that have the sgRNA plasmid along with the chromosomally integrated dcas9 cassette will be CamR+, KanR+.
Data analysis and graphing
GraphPad Prism Version 10.1 for Windows, GraphPad Software, (www.graphpad.com). One-way analysis of variance (ANOVA) was performed on multiple groups comparisons. Significance in the ANOVA analysis was followed by the use of between-group comparisons calculated using the Tukey’s Post Hoc comparison test. One-way or two-way ANOVAs and unpaired t-tests were used to analyze data for each graph as noted. For two-way ANOVA analyses, Šídák’s multiple comparisons test was used.
Euprymna scolopes husbandry and hatchling inoculation protocols
E. scolopes husbandry and hatchling inoculation protocols are detailed in Supplementary File 1. The Nishiguchi laboratory’s squid husbandry room at UC Merced is IACUC certified for all related experiments.
Luminescence measurement and quantification of symbiotic bacteria in LO
Luminescence produced by V. fischeri colonized hatchlings was measured with a Turner Designs TD20/20 luminometer (Turner Design). Juveniles were placed in 5 mL of sterile artificial seawater in 30 mL glass scintillation vials, and light produced was recorded for 15 s. Triplicate measurements (arbitrary light units, LU, per animal) were obtained and averaged.
The number of V. fischeri cells colonizing the squid light organ were assayed using LBS agar plates. Individual hatchlings to be assayed (typically after having their luminescence reading taken) were rinsed three times in 5 mL of sterilized artificial seawater. Juveniles were homogenized in a 1.5 mL Eppendorf microtubes using sterile 1.5 mL plastic pestles (Bel-Art) with 0.5 mL of sterile artificial seawater. 100 μL of the homogenate was used after serial dilution to plate in triplicate on LBS agar plates incubated at 28 °C. At 24 h colonies were counted to determine the colony forming units/light organ (CFU/LO).
In vitro luminescence, fluorescence, and growth.
Strains were grown with shaking at 28 °C in SWT, with and without CRISPRi induction. At regular intervals, 1 mL aliquots of each culture were assayed for cell density (Optical density (OD600) via absorbance at 600 nm) with 1 mL cuvettes in a UV-3100PC spectrophotometer (VWR) and 5 mL aliquots in 30 mL glass scintillation vials were measured for luminescence (relative light units, RLU) in a Turner Designs TD-20/20 luminometer. A SpectraMax iD5 multimodal platereader was also used for measuring OD600, and for bioluminescence production. Fluorescence of mRFP tagged CRISPRi strains could also be quantified (ext: 590, emm: 612), and read in conjunction with OD600 and luminescence.
Motility assays
For motility repression experiments, V. fischeri ES114 (control) and V. fischeri CRISPRi strains targeting flrA were grown overnight in TBS broth (1% tryptone, 2% sodium chloride) at 28 °C, with and without IPTG inducer supplementation as indicated, then sub-cultured in the same medium for 2 h at 28 °C (static). Cells to be assayed were normalized to an OD600 of 0.2 and 10 μL were drop spotted onto TBS soft agar plates with and without IPTG inducer supplementation as indicated, and incubated at 28 °C. Migration distance was measured hourly (as diameter to outer motility ring, mm) and plotted versus time.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank E.V. Stabb, K.L. Visick, and A. Dunn for supplying various plasmids for our study.
Author contributions
BLP and MKN conceived of this study. BLP and MKN designed the experiments, and BLP performed the experiments. BLP performed the data analysis. BLP wrote the manuscript. MKN contributed to writing and editing the manuscript. The authors read and approved the final manuscript.
Funding
The work was supported by NSF DBI-2214028, NASA EXO 80NSSC18K1053 and the School of Natural Sciences at UC Merced to M.K.N.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
The UC Merced Nishiguchi squid lab follows animal ethics protocols designed specifically for cephalopod use under our IACUC (Institutional Animal Care and Use Committee). All users must have animal welfare certifications in order to work with our research animals. The Nishiguchi squid laboratory is certified by our IACUC (Institutional Animal Care and Use Committee) at UC Merced.
Consent for publication
The participant has consented to the submission of this article to the journal. We confirm that the manuscript, or part of it, has neither been published nor is currently under consideration for publication. All co-authors approved this work and the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alker AT, Farrell MV, Aspiras AE, Dunbar TL, Fedoriouk A, Jones JE, Mikhail SR et al (2023) A modular plasmid toolkit applied in marine bacteria reveals functional insights during bacteria-stimulated metamorphosis. Mbio 14(4):e01502-e1523. 10.1128/mbio.01502-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschtgen MS, Brennan CA, Nikolakakis K, Cohen S, McFall-Ngai M, Ruby EG (2019) Insights into flagellar function and mechanism from the squid-vibrio symbiosis. Npj Biofilms Microbiomes 5(1):1–10. 10.1038/s41522-019-0106-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta AB, Enright AL, Siletti C, Peters JM (2020a) A high-efficacy CRISPR interference system for gene function discovery in Zymomonas Mobilis. Appl Environ Microbiol 86(23):e01621-e1720. 10.1128/AEM.01621-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta AB, Ward RD, Tran JS, Bacon EE, Peters JM (2020b) Programmable gene knockdown in diverse bacteria using mobile-CRISPRi. Curr Protoc Microbiol. 10.1002/cpmc.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K, Pedersen LE, Weber T, Lee SY (2016) CRISPy-web: an online resource to design sgRNAs for CRISPR applications. Synth Syst Biotechnol 1(2):118–121. 10.1016/j.synbio.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin K, Shaw S, Tong Y, Weber T (2020) Designing sgRNAs for CRISPR-BEST Base Editing Applications with CRISPy-Web 2.0. Synthe Syst Biotechnol 5(2):99–102. 10.1016/j.synbio.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne DG, Morrow KM, Webster NS (2016) Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems. Annu Rev Microbiol 70(1):317–340. 10.1146/annurev-micro-102215-095440 [DOI] [PubMed] [Google Scholar]
- Brooks JF, Gyllborg MC, Cronin DC, Quillin SJ, Mallama CA, Foxall R, Whistler C, Goodman AL, Mandel MJ (2014) Global discovery of colonization determinants in the squid symbiont Vibrio fischeri. Proc Natl Acad Sci USA 111(48):17284–17289. 10.1073/pnas.1415957111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun G, Yang J, Seo SW (2023) CRISPRi-mediated Tunable control of gene expression level with engineered single-guide RNA in Escherichia coli. Nucleic Acids Res 51(9):4650–4659. 10.1093/nar/gkad234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro F, Place NM, Mekalanos JJ (2019) Analysis of lipoprotein transport depletion in Vibrio cholerae using CRISPRi. Proc Natl Acad Sci USA 116(34):17013–17022. 10.1073/pnas.1906158116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Phan M-D, Schembri MA (2024) Modified Tn7 transposon vectors for controlled chromosomal gene expression. Appl Environ Microbiol 90(10):e01556-e1624. 10.1128/aem.01556-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Dozal A, Hogan D, Gorman C, Quintanal-Villalonga A, Nishiguchi MK (2012) Multiple Vibrio fischeri genes are involved in biofilm formation and host colonization. FEMS Microbiol Ecol 81(3):562–573. 10.1111/j.1574-6941.2012.01386.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Dozal AA, Clayton Gorman C, Lostroh P, Nishiguchi MK (2014) Gene-swapping mediates host specificity among symbiotic bacteria in a beneficial symbiosis. PLoS ONE 9(7):e101691. 10.1371/journal.pone.0101691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DG, Tepavčević J, Visick KL (2020) Genetic manipulation of Vibrio fischeri. Curr Protoc Microbiol 59(1):e115. 10.1002/cpmc.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collias D, Beisel CL (2021) CRISPR technologies and the search for the PAM-free nuclease. Nat Commun 12(1):555. 10.1038/s41467-020-20633-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Vigouroux A, Rousset F, Varet H, Khanna V, Bikard D (2018) A CRISPRi screen in E. coli reveals sequence-specific toxicity of dCas9. Nat Commun 9:1912. 10.1038/s41467-018-04209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLoney CR, Bartley TM, Visick KL (2002) Role for phosphoglucomutase in Vibrio fischeri-Euprymna scolopes symbiosis. J Bacteriol 184(18):5121–5129. 10.1128/JB.184.18.5121-5129.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didovyk A, Borek B, Hasty J, Tsimring L (2016) Orthogonal modular gene repression in E. coli using engineered CRISPR/Cas9. ACS Synth Biol 5(1):81–88. 10.1021/acssynbio.5b00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap PV, Kuo A (1992) Cell density-dependent modulation of the Vibrio fischeri luminescence system in the absence of autoinducer and LuxR protein. J Bacteriol 174(8):2440–2448. 10.1128/jb.174.8.2440-2448.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AK, Martin MO, Stabb EV (2005) Characterization of pES213, a small mobilizable plasmid from Vibrio fischeri. Plasmid 54(2):114–134. 10.1016/j.plasmid.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Dunn AK, Millikan DS, Adin DM, Bose JL, Stabb EV (2006) New Rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl Environ Microbiol 72(1):802–810. 10.1128/AEM.72.1.802-810.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A, Burlingame AL, Eberhard C, Kenyon GL, Nealson KH, Oppenheimer NJ (1981) Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 20(9):2444–2449. 10.1021/bi00512a013 [DOI] [PubMed] [Google Scholar]
- Engebrecht J, Silverman M (1984) Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci USA 81(13):4154–4158. 10.1073/pnas.81.13.4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J, Nealson K, Silverman M (1983) Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32(3):773–781. 10.1016/0092-8674(83)90063-6 [DOI] [PubMed] [Google Scholar]
- Enright AL, Heelan WJ, Ward RD, Peters JM (2024) CRISPRi functional genomics in bacteria and its application to medical and industrial research. Microbiol Mol Biol Rev 88(2):e00170-e222. 10.1128/mmbr.00170-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Guo J, Wang T, Zhang C, Xing X-H (2021) Guide-target mismatch effects on dCas9–sgRNA binding activity in living bacterial cells. Nucleic Acids Res 49(3):1263–1277. 10.1093/nar/gkaa1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidopiastis PM, Childs C, Esin JJ, Stellern J, Darin A, Lorenzo A, Mariscal VT et al (2024) Corrected and republished from: ‘Vibrio fischeri possesses Xds and Dns nucleases that differentially influence phosphate scavenging, aggregation, competence, and symbiotic colonization of squid’. Appl Environ Microbiol 90(6):e00328-e424. 10.1128/aem.00328-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana J, Dong C, Ham JY, Zalatan JG, Carothers JM (2018) Regulated expression of sgRNAs tunes CRISPRi in E. coli. Biotechnol J 13(9):1800069. 10.1002/biot.201800069 [DOI] [PubMed] [Google Scholar]
- Geyman LJ, Tanner MP, Rosario-Melendez N, Peters JM, Mandel MJ, van Kessel JC (2024) Mobile-CRISPRi as a powerful tool for modulating Vibrio gene expression. Appl Environ Microbiol 90(6):e0006524. 10.1128/aem.00065-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J, Dunlap PV, Ruby EG (1994) Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J Bacteriol 176(22):6986–6991. 10.1128/jb.176.22.6986-6991.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Li Y, Hong W, Lin M (2024) A robust CRISPR interference gene repression system in Vibrio parahaemolyticus. Arch Microbiol 206(1):41. 10.1007/s00203-023-03770-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 337(6096):816–821. 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson N, Mekalanos JJ (2000) TnAraOut, a transposon-based approach to identify and characterize essential bacterial genes. Nat Biotechnol 18(7):740–745. 10.1038/77305 [DOI] [PubMed] [Google Scholar]
- Kim SK, Seong W, Han GH, Lee D-H, Lee S-G (2017) CRISPR interference-guided multiplex repression of endogenous competing pathway genes for redirecting metabolic flux in Escherichia coli. Microb Cell Fact 16:188. 10.1186/s12934-017-0802-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch EJ, Miyashiro T, McFall-Ngai MJ, Ruby EG (2014) Features governing symbiont persistence in the squid-Vibrio association. Mol Ecol 23(6):1624–1634. 10.1111/mec.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, Qi LS (2013) CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc 8(11):2180–2196. 10.1038/nprot.2013.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Ostrov N, Wong BG, Gold MA, Khalil AS, Church GM (2019) Functional genomics of the rapidly replicating bacterium Vibrio natriegens by CRISPRi. Nat Microbiol 4(7):1105–1113. 10.1038/s41564-019-0423-8 [DOI] [PubMed] [Google Scholar]
- Lin H, Min Yu, Wang X, Zhang X-H (2018) Comparative genomic analysis reveals the evolution and environmental adaptation strategies of vibrios. BMC Genomics 19:135. 10.1186/s12864-018-4531-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gallay C, Kjos M, Domenech A, Slager J, van Kessel SP, Knoops K et al (2017) High-throughput CRISPRi phenotyping identifies new essential genes in Streptococcus pneumoniae. Mol Syst Biol 13(5):931. 10.15252/msb.20167449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ML, Mullis AS, Leenay RT, Beisel CL (2015) Repurposing endogenous type I CRISPR-Cas systems for programmable gene repression. Nucleic Acids Res 43(1):674–681. 10.1093/nar/gku971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistro G, Magistro C, Stief CG, Schubert S (2018) A simple and highly efficient method for gene silencing in Escherichia coli. J Microbiol Methods 154:25–32. 10.1016/j.mimet.2018.10.003 [DOI] [PubMed] [Google Scholar]
- Milewski S (2002) Glucosamine-6-phosphate synthase—the multi-facets enzyme. Biochim Biophys Acta 1597(2):173–192. 10.1016/S0167-4838(02)00318-7 [DOI] [PubMed] [Google Scholar]
- Millikan DS, Ruby EG (2003) FlrA, a Σ54-dependent transcriptional activator in Vibrio fischeri, is required for motility and symbiotic light-organ colonization. J Bacteriol 185(12):3547–3557. 10.1128/jb.185.12.3547-3557.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimee M, Tucker AC, Voigt CA, Timothy KLu (2015) Programming a human commensal bacterium, Bacteroides thetaiotaomicron, to sense and respond to stimuli in the murine gut microbiota. Cell Syst 1(1):62–71. 10.1016/j.cels.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro T, Ruby EG (2012) Shedding light on bioluminescence regulation in Vibrio fischeri. Mol Microbiol 84(5):795–806. 10.1111/j.1365-2958.2012.08065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem M, Majeed S, Hoque MZ, Ahmad I (2020) Latest developed strategies to minimize the off-target effects in CRISPR-Cas-mediated genome editing. Cells 9(7):1608. 10.3390/cells9071608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima N, Goh S, Good L, Tamura T (2012) Multiple-gene silencing using antisense RNAs in Escherichia coli. Methods Mol Biol 815:307–319. 10.1007/978-1-61779-424-7_23 [DOI] [PubMed] [Google Scholar]
- Nijvipakul S, Wongratana J, Suadee C, Entsch B, Ballou DP, Chaiyen P (2008) LuxG is a functioning flavin reductase for bacterial luminescence. J Bacteriol 190(5):1531–1538. 10.1128/jb.01660-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norsworthy A, Visick K (2013) Gimme shelter: how Vibrio fischeri successfully navigates an animal’s multiple environments. Front Microbiol. 10.3389/fmicb.2013.00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourabadi N, Nishiguchi MK (2021) pH adaptation drives diverse phenotypes in a beneficial bacterium-host Mutualism. Front Ecol EvoL. 10.3389/fevo.2021.611411 [Google Scholar]
- O’Grady EA, Wimpee CF (2008) Mutations in the lux operon of natural dark mutants in the genus Vibrio. Appl Environ Microbiol 74(1):61–66. 10.1128/AEM.01199-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea TM, DeLoney-Marino CR, Shibata S, Aizawa S-I, Wolfe AJ, Visick KL (2005) Magnesium promotes flagellation of Vibrio fischeri. J Bacteriol 187(6):2058–2065. 10.1128/JB.187.6.2058-2065.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondrey JM, Visick KL (2014) Engineering Vibrio fischeri for inducible gene expression. Open Microbiol J 8:122–129. 10.2174/1874285801408010122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M, Schwartzman JA, Dunn AK, Lu Z, Ruby EG (2015) A Single host-derived glycan impacts key regulatory nodes of symbiont metabolism in a coevolved mutualism. Mbio. 10.1128/mbio.00811-15.10.1128/mbio.00811-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JE, Craig NL (2001) Tn7: smarter than we thought. Nat Rev Mol Cell Biol 2(11):806–814. 10.1038/35099006 [DOI] [PubMed] [Google Scholar]
- Peters JM, Colavin A, Shi H, Czarny TL, Larson MH, Wong S, Hawkins JS et al (2016) A comprehensive, CRISPR-based functional analysis of essential genes in bacteria. Cell 165(6):1493–1506. 10.1016/j.cell.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Koo B-M, Patino R, Heussler GE, Hearne CC, Jiuxin Qu, Inclan YF et al (2019) Enabling genetic analysis of diverse bacteria with mobile-CRISPRi. Nat Microbiol 4(2):244–250. 10.1038/s41564-018-0327-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipes BL, Nishiguchi MK (2022) Nocturnal acidification: a coordinating cue in the Euprymna scolopes–Vibrio fischeri symbiosis. Int J Mol Sci 23(7):3743. 10.3390/ijms23073743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack-Berti A, Wollenberg MS, Ruby EG (2010) Natural transformation of Vibrio fischeri requires tfoX and tfoY. Environ Microbiol 12(8):2302–2311. 10.1111/j.1462-2920.2010.02250.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA (2013) Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152(5):1173–1183. 10.1016/j.cell.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachwalski K, Tu MM, Madden SJ, French S, Hansen DM, Brown ED (2024) A mobile CRISPRi collection enables genetic interaction studies for the essential genes of Escherichia coli. Cell Reports Methods 4(1):100693. 10.1016/j.crmeth.2023.100693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis AC, Halper SM, Vezeau GE, Cetnar DP, Hossain A, Clauer PR, Salis HM (2019) Simultaneous repression of multiple bacterial genes using nonrepetitive extra-long sgRNA arrays. Nat Biotechnol 37(11):1294–1301. 10.1038/s41587-019-0286-9 [DOI] [PubMed] [Google Scholar]
- Rock JM, Hopkins FF, Chavez A, Diallo M, Chase MR, Gerrick ER, Pritchard JR et al (2017) programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat Microbiol 2(4):1–9. 10.1038/nmicrobiol.2016.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F, Cui L, Siouve E, Becavin C, Depardieu F, Bikard D (2018) Genome-wide CRISPR-dCas9 screens in E. coli identify essential genes and phage host factors. PLoS Genet 14(11):e1007749. 10.1371/journal.pgen.1007749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby EG, Asato LM (1993) Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch Microbiol 159(2):160–167. 10.1007/BF00250277 [DOI] [PubMed] [Google Scholar]
- Schwartzman JA, Koch E, Heath-Heckman EAC, Zhou L, Kremer N, McFall-Ngai MJ, Ruby EG (2015) The chemistry of negotiation: rhythmic, glycan-driven acidification in a symbiotic conversation. Proc Natl Acad Sci 112(2):566–571. 10.1073/pnas.1418580112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Septer AN, Stabb EV (2012) Coordination of the arc regulatory system and pheromone-mediated positive feedback in controlling the Vibrio fischeri lux operon. PLoS ONE 7:49590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha P, Razvi A, Fung BL, Eichinger SJ, Visick KL (2022) Mutational analysis of Vibrio fischeri C-Di-GMP-modulating genes reveals complex regulation of motility. J Bacteriol 204(7):e00109-e122. 10.1128/jb.00109-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabb EV, Ruby EG (2002) RP4-based plasmids for conjugation between Escherichia coli and members of the Vibrionaceae. Methods Enzymol. 10.1016/S0076-6879(02)58106-4 [DOI] [PubMed] [Google Scholar]
- Stabb EV, Visick KL (2014) Chapter 20—Vibrio Fischeri: a bioluminescent light-organ symbiont of the bobtail squid Euprymna scolopes. In: DeLong EF, Stackebrandt E, Lory S, Thompson F (eds) The prokaryotes. Springer-Verlag, Berlin [Google Scholar]
- Sunagawa S, Coelho LP, Chaffron S, Kultima JR, Labadie K, Salazar G, Djahanschiri B et al (2015) Structure and function of the global ocean microbiome. Science 348(6237):1261359. 10.1126/science.1261359 [DOI] [PubMed] [Google Scholar]
- Tan SZ, Reisch CR, Prather KLJ (2018) A robust CRISPR interference gene repression system in pseudomonas. J Bacteriol. 10.1128/jb.00575-17.10.1128/jb.00575-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FL, Cleenwerck I, Swings J, Matsuyama J, Iida T (2007) Genomic diversity and homologous recombination in Vibrio parahaemolyticus as revealed by amplified fragment length polymorphism (AFLP) and multilocus sequence analysis (MLSA). Microbes Environ 22(4):373–379. 10.1264/jsme2.22.373 [Google Scholar]
- Todor H, Silvis MR, Osadnik H, Gross CA (2021) Bacterial CRISPR screens for gene function. Curr Opin Microbiol 59:102–109. 10.1016/j.mib.2020.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SC, Miyashiro T (2013) Quorum sensing in the squid-Vibrio symbiosis. Int J Mol Sci 14:16386–16401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigouroux A, Bikard D (2020) CRISPR tools to control gene expression in bacteria. Microbiol Mol Biol Rev 84(2):e00077-e119. 10.1128/MMBR.00077-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan N, Lema KA, Nedved BT, Hadfield MG (2019) Microbiomes of the polychaete Hydroides elegans (Polychaeta: Serpulidae) across its life-history stages. Mar Biol 166(2):19. 10.1007/s00227-019-3465-9 [Google Scholar]
- Visick KL (2009) An intricate network of regulators controls biofilm formation and colonization by Vibrio fischeri. Mol Microbiol 74:782–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL, Foster J, Doino J, McFall-Ngai M, Ruby EG (2000) Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J Bacteriol 182(16):4578–4586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL, Hodge-Hanson KM, Tischler AH, Bennett AK, Mastrodomenico V (2018) Tools for rapid genetic engineering of Vibrio fischeri. Appl Environ Microbiol 84(14):e00850-e918. 10.1128/AEM.00850-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL, Ruby EG (1997) New genetic tools for use in the marine bioluminescent bacterium Vibrio fischeri. Bioluminescence and chemiluminescence: 119–22.
- Wolfe AJ, Millikan DS, Campbell JM, Visick KL (2004) Vibrio fischeri s54 controls motility, biofilm formation, luminescence, and colonization. Appl Environ Microbiol 70(4):2520–2524. 10.1128/AEM.70.4.2520-2524.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Visick KL (2009) Vibrio biofilms so much the same yet so different. Trends Microbiol 17:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Sun Y, Peters JM, Gross CA, Garner EC, Helmann JD (2016) Depletion of undecaprenyl pyrophosphate phosphatases disrupts cell envelope biogenesis in Bacillus subtilis. J Bacteriol 198(21):2925–2935. 10.1128/jb.00507-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.