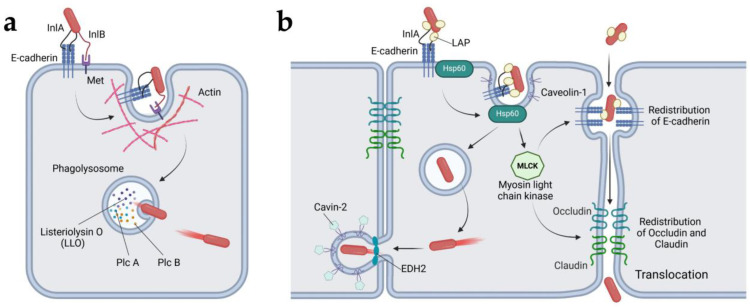
Figure 4.
Mechanisms of adhesion, invasion, and translocation of epithelial cells by L. monocytogenes. (a) L. monocytogenes binds to epithelial cell receptors E-cadherin and the Met via InlA and InlB. This interaction triggers receptor-mediated endocytosis of L. monocytogenes. Once inside the cell, L. monocytogenes uses LLO, PlcA, and PlcB to escape from the endocytic vesicle into the cytoplasm. (b) The Listeria adhesion protein (LAP) interacts with the epithelial cell surface receptor Hsp60, facilitating access of InlA to basolateral E-cadherin. This interaction triggers caveolin-1-mediated endocytosis, activates MLCK, and disrupts the intestinal cell-cell barrier by redistributing junctional proteins such as claudin-1, occludin, and E-cadherin, ultimately enabling bacterial translocation across the epithelium. InlA, internalin A; InlB, internalin B; E-cadherin, epithelial cadherin; LLO, listeriolysin O; LAP, Listeria adhesion protein; Hsp60, heat shock protein 60; Met, MET receptor tyrosine kinase; PlcA, phosphatidylinositol-specific phospholipase; PlcB, broad-range phospholipase C, MLCK, myosin light chain kinase. Created with BioRender.com.