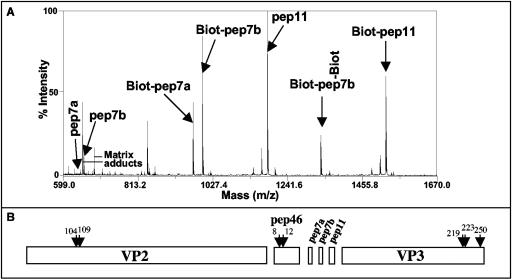
FIG. 5.
Peptide accessibility to biochemical labeling and protease. (A) IBDV particles were submitted to biotinylation before mass spectrometry analysis. A mass/charge window ranging from 599 to 1,670 is presented. The peptides pep7a, pep7b, and pep11 with their biotinylated forms were observed. Because of the presence of a lysine in the pep7a sequence, two biotinylated forms of pep7b were detected. pep46 and VP3 were not biotinylated in these experimental conditions. (B) IBDV particles were submitted to trypsin digestion, and the generated peptides were analyzed by mass spectrometry. The peptides were assigned to the primary sequences of the main structural components of the virions VP2 and VP3 and the peptides pep46, pep7a, pep7b, and pep11. The numbers and arrows indicate amino acid positions that are located immediately upstream the cleavage sites. The results presented were obtained after 1 minute of coincubation of the virus with trypsin. Cleaved products of pep46 were also observed for longer coincubation times.