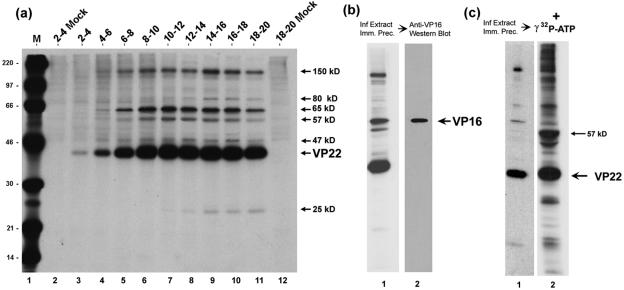
FIG. 2.
Analysis of VP22 interacting species by immunoprecipitation. (a) HeLa cells were infected (multiplicity of infection, 5) or mock-infected and pulse-labeled with 20 μCi/ml [35S]methionine for the times shown above each lane (hours). Soluble extracts were prepared and VP22 was immunoprecipitated with anti-VP22 antibody AGV30. Equivalent samples were separated by SDS-PAGE and exposed for autoradiography. The position of VP22 and the VP22-associated proteins is shown to the right of the panel. Lane M, 14C-labeled protein size markers with masses shown to the left of the panel in kDa. (b) VP22 was immunoprecipitated with AGV30 from infected-cell extracts labeled with 20 μCi/ml [35S]methionine from 12 to 16 h postinfection. The samples were separated and exposed for autoradiography as before. A duplicate gel was electroblotted onto nitrocellulose and the blot probed with anti-VP16 monoclonal antibody (LP1). VP16 was detected by Western blotting of the VP22 immunoprecipitate and comigrated with the 65-kDa band detected by autoradiography. (c) A kinase activity immunoprecipitates with VP22. Autoradiograph of the immunoprecipitation of VP22 from infected-cell extracts performed as in panel a. Duplicate immunoprecipitation reactions were equilibrated in kinase buffer and [γ-32P]ATP. Following incubation at 37°C for 15 min the proteins were solubilized in SDS lysis buffer, separated on SDS-PAGE, and exposed for autoradiography of 32-P labeled bands. The positions of VP22 and the major in vitro phosphorylation product are shown the right of the panel.