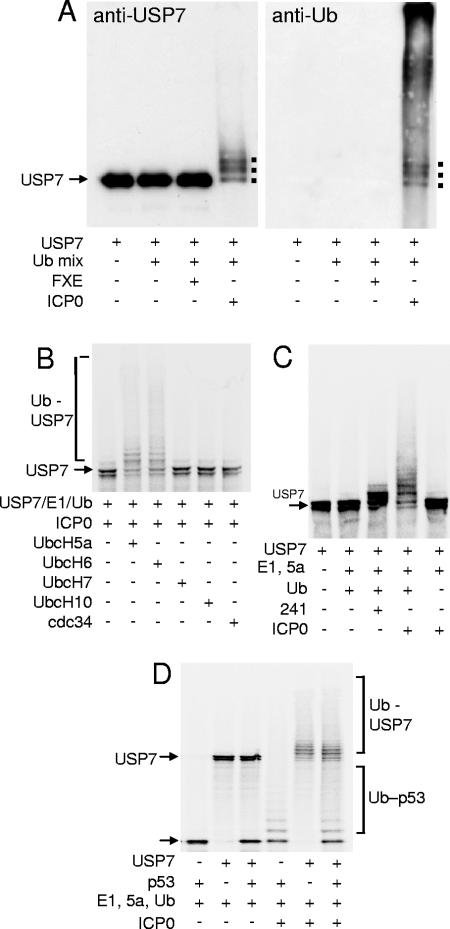
FIG. 1.
ICP0 ubiquitinates USP7 in vitro. (A) Purified USP7 was incubated with E1, UbcH5a, and ubiquitin (Ub mix) in a buffer containing ATP and with purified samples of either wild-type ICP0 or its RING finger deletion derivative FXE. The products were analyzed by Western blotting using either anti-USP7 (left) or antiubiquitin (right) antibodies. Ubiquitinated forms of USP7 form a higher-molecular-weight smear, with distinct bands corresponding to USP7 molecules with small numbers of ubiquitin adducts marked by dots. These bands were also detected by the antiubiquitin antibody. (B) ICP0 ubiquitinates radiolabeled USP7 in conjunction with E2 enzymes UbcH5a and UbcH6, but not UbcH7, UbcH10, or cdc34 (UbcH3). The relevant E2 enzymes were added to mixtures containing the other components as marked. (C) USP7 is not efficiently ubiquitinated by the isolated RING finger domain of ICP0. Radiolabeled USP7 was mixed with E1, UbcH5a (5a), ubiquitin (Ub), and either GST-241 (241; the N-terminal third of ICP0 containing the RING finger linked to GST) or full-length ICP0. (D) ICP0 ubiquitinates both USP7 and p53 in vitro. Radiolabeled USP7 and p53 were incubated with components of ICP0 ubiquitination reactions as marked.