Abstract
Background
Bread wheat (Triticum aestivum) is one of the most widely cultivated crops globally; it is nutritionally demanding and may be responsible for soil exhaustion, requiring adequate fertilization to maintain high yields and grain quality. Targeted supplementation of macro- and micronutrients can also be used for the agronomic biofortification of the grains. However, excessive chemical fertilizers can harm the environment and human health, and more sustainable options are therefore required. This work proposes alternative strategies to chemical fertilization, including applying organic fertilizers, biostimulants, and low-impact agronomical practices like foliar spraying, to achieve high yields and enrichment in cationic nutrients calcium, magnesium, and potassium.
Experimental plan
The study investigates the impact of different fertilization strategies on wheat yield and nutrient composition using two wheat genotypes characterized by different nitrogen (N) grain content. The plants were grown in pots and underwent differential root fertilization with 50 kg ha−1 N at the tillering stage, comparing mineral and organic products. At heading, foliar treatments (25 kg ha−1 N) were applied, comparing a traditional urea supplementation with a combination of biostimulants from organic wastes and calcium, magnesium and potassium nitrates. The plants were analyzed for their health and the expression of genes for nutrient homeostasis during growth, and for yield and grain quality at harvesting.
Results
The two alternative fertilization approaches positively impacted plant health and yield in both cultivars. Root fertilization accounted for most of the total variance, affecting both early and late-stage yield components; the organic fertilizer produced results comparable to those of the mineral one. Furthermore, the foliar application of base cations and biostimulants led to beneficial changes in nutrient homeostasis and grain mineral content, although the increase in calcium, magnesium and potassium was moderate and genotype-specific.
Conclusions
This work identifies organic amendments, foliar spraying and biostimulants as alternative and sustainable strategies that can be as effective as chemical fertilization in improving wheat plant health, yield and grain composition. On the other hand, supplementing with cation nutrients at heading showed minimal biofortification benefits. The study emphasizes the importance of considering genotype-specific needs to optimize nutrient uptake and yield across different wheat cultivars.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-025-06804-3.
Keywords: Biostimulants, Wheat, Foliar fertilization, Nutritional value
Introduction
Wheat is the second most important cereal for human consumption (ca. 67 kg person−1 year−1) after rice and feed production after maize (data from FAOSTAT for the year 2021: www.fao.org/faostat/). This crop, and in particular bread wheat (Triticum aestivum L.), is one of most widely cultivated worldwide, ranking first in cultivated area (more than 220 million ha) and fourth for annual production (above 770 million t). Although T. aestivum is relatively adaptable and widespread worldwide, its yield is strongly influenced by cultural conditions and varies widely in different geographical areas (ranging from 0.4–10 t ha−1, FAOSTAT 2021), due to both climatic differences (e.g., temperature, humidity, seasonal cycling) and the availability of agricultural inputs [1–4].
Thanks to the long history of wheat breeding, wheat grain has a relatively high protein content, even though significant variability (7–22% dry weight, DW, [5]; 13–20% DW, [6]) was identified and selected for different uses in the food and feed industry [7, 8]. For this reason, wheat has high nutritional needs in terms of both nitrogen (N) and other nutrients, which are consistently taken up during wheat cultivation, a fact that allowed its acknowledgement as a soil-impoverishing crop [9]. Indeed, wheat was estimated to remove up to 200 kg ha−1 N and potassium (K) from soil yearly, as well as 50 kg ha−1 phosphorus (P) [10, 11]. Given this evidence, adequate wheat fertilization is necessary for high yields and good grain quality [12–15] to avoid soil exhaustion. On the other hand, massive chemical fertilization is known to impact the environment negatively, impairing soil, water and air quality and adversely affecting human health [16, 17]. To counteract chemical fertilization as an ongoing negative trend, several alternatives have been proposed to sustainably deliver nutrients to plants, such as organic or slow-release fertilizers, amendments, and biostimulants, as well as the application of low-impact agricultural practices, including, for example, foliar spraying [16, 18–20]. Among the former, biostimulants, i.e., a variety of products able to improve plant productivity by processes not directly ascribable to plant growth regulation [21, 22], have raised attention in recent years for their peculiar properties. Recent research has associated biostimulants with increased yield and grain quality in wheat, as well as amelioration of nutrient homeostasis and improved response to stresses [23–27]. In the view of a sustainable and circular economy, it has been demonstrated that effective biostimulants, similarly to organic fertilizers, can be obtained from the appropriate conversion of organic wastes of the agri-food and industrial production chains, contributing to environmental and economic sustainability [28]. As for agricultural practices, foliar spraying is considered a valid alternative to enhance plant nutrition: promising results have been obtained in this direction in cereals and other crops [29, 30], taking into account several advantages of the technique, such as reduced leakage of nutrients, limited accumulation of excess ions in the soil, and increased efficiency in nutrient uptake [31, 32]. Weaknesses of this practice are also worth noticing: foliar nutrients generally have low penetration due to the barrier properties of cuticles and other epidermal structures, and fertilizer solubility, together with developmental and environmental factors, can hinder the efficacy of nutrient translocation in leaves [31–33]. Therefore, this type of treatment has been proposed as effective when in combination with traditional soil fertilization to correct plant nutritional disorders, promote plant growth and yield, and enrich edible plant tissues with mineral nutrients that are beneficial for human health [31–33]. The latter, known as agronomic biofortification, has been widely proposed in wheat. Studies have mostly regarded micronutrients such as iron (Fe) and zinc (Zn), which are among the most common deficiencies in the so-called “hidden hunger” [34–36]. However, deficiency in base cation nutrients calcium (Ca), magnesium (Mg) and K is also widespread and needs to be addressed [37]. Indeed, evidence supports that Ca, Mg, and K intake has beneficial effects in lowering the potential renal acid load (PRAL), a dietary parameter that estimates the impact of food on urine pH. High PRAL values are associated with a diet high in proteins and poor in base cations and have been correlated with a higher incidence of clinical conditions such as osteoporosis, hypertension and chronic kidney disease; cereals and derived products have a positive (i.e., acidic) PRAL [38, 39]. In this view, identifying effective strategies for the agronomical biofortification of wheat with Ca, Mg, and K is a current concern; however, research in this field has been limited, with moderately positive results [40].
The focus of this work was to propose viable alternatives to traditional chemical fertilization, aiming to reach equal or superior wheat health, nutrient efficiency and yield; at the same time, the research wanted to achieve higher grain quality by supplementing base cationic nutrients. In a greenhouse-scale experiment, two different wheat genotypes, Rebelde and Bagou, were subjected first to root fertilization at tillering, followed by a foliar treatment at heading. At the root level, a mineral fertilizer and an organic amendment derived from manure were compared for their effect on plant health and macronutrient homeostasis. Foliar treatments during heading included the application of N either as urea or as nitrates (Ca(NO3)2, Mg(NO3)2 and KNO3), either in the presence or absence of biostimulants from production wastes. The final effect of the different strategies and the contribution of the different treatments was evaluated in terms of plant growth, yield, and mineral content of the grain; the expression of nutrient transporters was also considered to further elucidate the plants’ nutritional status and mineral homeostasis. The results highlight the possibility of adopting alternative strategies for wheat fertilization, allowing a reduced impact on the environment and the concurrent recycling of organic wastes.
Material and methods
Experimental setup and treatments
The experiment was conducted on two common winter wheat (T. aestivum L.) cultivars, Rebelde and Bagou. Rebelde (seeds were obtained at APSOV Sementi, Pavia, Italy—https://www.apsovsementi.com) is a superior bread-making wheat genotype with high protein content; Bagou (seeds were obtained at RV Venturoli, Bologna, Italy—https://www.rv-venturoli.com) is a soft wheat genotype with low protein content, used mainly for biscuits, crackers and breakfast foods. Seeds were pre-treated with the Celest Trio fungicide (Syngenta, Basel, Switzerland). Sowing was performed in 5 L pots (diameter 21 cm, height 17.5 cm) filled with 3 kg of a 1:1 mixture of garden soil (Klasmann-Deilmann GmbH, Geeste, Germany; specifics are reported in Supplementary Material and Methods) and silica sand; the garden soil contained already a basal NPK fertilization (N:P:K 14:10:18, 0.8 kg m−3). Seeds were sown on 15 December 2020, maintaining row distribution and seed density as in field conditions (0.12 m apart rows and 220 kg ha−1 seeds; [40]). Each pot, containing 22 plants, represented a replicate. Plants were vernalized in the field from January 2021 until April 2021, then moved into a greenhouse under natural light conditions, with a minimum temperature of 22 °C and about 60% relative humidity; pots were randomized every 15 days to avoid the edge effect. Uniformity of water regime was controlled by supplementing the same volume of water (100 mL during winter, 150 mL during spring and 200 mL from May to June) to each pot, twice per week until the end of the experiment, to a total of 8700 mL of water in each pot. The amount of water was decided to exclude percolation from the pots, in consideration of the different seasonal needs of the plants.
Nutrients were provided as split fertilization in three different growth stages, according to the common practices of wheat cultivation in Northern Italy and considering an intermediate level of N supplementation in consideration of the different characteristics of the two genotypes used [41, 42]. Firstly, a basal NPK fertilization (N:P:K 14:10:18, 0.8 kg m−3) was provided pre-sowing to all pots as included in the growth substrate. Secondly, root fertilization (50 kg ha−1 N) was performed at tillering using N from different origins, all provided as pelleted solid fertilizer. Lastly, foliar supplementation (25 kg ha−1 N) was applied at heading, providing N in different forms, with or without the addition of biostimulants. A schematic representation of the different fertilization strategies, each applied to both wheat genotypes, is reported in Fig. 1. The root and foliar strategies are detailed in the next paragraphs.
Fig. 1.
Experimental plan. Schematic representation of the experimental thesis applied in this work. Root fertilization approaches, applied at tillering, are reported in columns and foliar treatments, performed at heading, in rows. The untreated control (UTC) received no nutrient supplementation
Root fertilization
Root fertilization was applied at tillering in three different forms, i.e., mineral P (MIN(P)), mineral N + P (MIN(N + P) and organic (ORG) (Table 1). Each treatment was provided to 18 pots and supplemented 17.8 kg ha−1 P; N addition was applied only for the MIN(N + P) and ORG conditions and consisted of 50 kg ha−1 N. Mineral treatments were applied as urea for N and soft rock phosphate for P. The organic treatment was provided as pelleted mature manure, produced by recovering and treating wastes of poultry and cattle farming; the contribution in terms of N and P was equal to the MIN(N + P) treatment. The chemical composition of the mineral and organic fertilizers is reported in Supplementary Material and Methods. The MIN(P) treatment was used as a non-N-treated control to evaluate the specific effect of N supplementation. Three pots, as untreated control (UTC), received no fertilization.
Table 1.
Treatments for root fertilization at tillering
| Treatment | Acronym | Composition |
|---|---|---|
| Mineral fertilizer with P | MIN(P) | 17.8 kg ha−1 P as soft rock phosphate (61 kg ha−1) |
| Mineral fertilizer with N and P | MIN(N + P) | 50 kg ha−1 N as urea (109 kg ha−1) + 17.8 kg ha−1 P as soft rock phosphate (61 kg ha−1) |
| Organic fertilizer | ORG | 50 kg ha−1 N and 17.8 kg ha−1 P as pelleted mature manure (592 kg ha−1) |
Foliar fertilization
Foliar fertilization was applied at early heading, supplementing 25 kg ha−1 N in a spraying water volume of 600 L ha−1. N was provided as urea in the UREA, UREA + AN and UREA + VEG treatments, and as Ca, Mg and K nitrates in the NO3, NO3 + AN and NO3 + VEG treatments (Table 2); in the nitrate treatments, nitrates were applied as proposed by the 40% dosage in [40]. Urea was added to equalize the N dosage. UREA + AN and NO3 + AN treatments were supplemented with 2.5 kg ha−1 of a biostimulant of animal origin, derived from the hydrolysis of bovine meat wastes. Similarly, UREA + VEG and NO3 + VEG treatments also contained 2.5 kg ha−1 of a biostimulant of vegetal origin, obtained by hydrolysis from residuals of castor oil (Ricinus communis) extraction. The specifics and chemical composition of the biostimulants are reported in Supplementary Material and Methods; biostimulant contribution to foliar N fertilization was negligible. Each foliar fertilization was applied to nine pots, three for each root treatment (MIN(P), MIN(N + P) and ORG). The UTC pots received no foliar fertilization in addition to no root treatment.
Table 2.
Treatments for foliar fertilization at heading
| Treatment | Acronym | Composition |
|---|---|---|
| Urea | UREA | 25 kg ha−1 N as urea (54.5 kg ha−1) |
| Urea + animal biostimulant | UREA + AN | 25 kg ha−1 N as urea + 2.5 kg ha−1 hydrolyzed bovine meat waste |
| Urea + vegetal biostimulant | UREA + VEG | 25 kg ha−1 N as urea + 2.5 kg ha−1 hydrolyzed castor-oil wastes |
| Ca, Mg and K nitrates | NO3 | 25 kg ha−1 N as nitrates + urea (38.8 kg ha−1 Ca(NO3)2, 45.6 kg ha−1 Mg(NO3)2, 81.7 kg ha−1 KNO3, 7.93 kg ha−1 urea) |
| Ca, Mg and K nitrates + animal biostimulant | NO3 + AN | 25 kg ha−1 N as in NO3 + 2.5 kg ha−1 hydrolyzed bovine meat waste |
| Ca, Mg and K nitrates + vegetal biostimulant | NO3 + VEG | 25 kg ha−1 N as in NO3 + 2.5 kg ha−1 hydrolyzed castor-oil wastes |
Plant sampling and measurements
To evaluate the effect of root fertilization, leaves were collected six weeks after root fertilization. The fourth leaf on the main stem was chosen for developmental uniformity. Leaves were pooled from five plants from three representative pots (three biological replicates) for each treatment, frozen in liquid nitrogen and stored for chlorophyll and gene expression analyses. To analyze the impact of foliar treatments, leaves were collected one week after spraying. Flag leaves were pooled from five plants for each pot from the same root and foliar fertilization (three biological replicates), then frozen in liquid nitrogen and stored for chlorophyll and gene expression analyses.
To evaluate yield and grain quality, ripe wheat heads were collected at the end of June 2021. Yield parameters included the number of spikes per plant (expressing tiller capacity), the number of seeds per spike, the weight of 1000 grains (TKW, thousand kernel weight) and the total yield. Each vase constituted a biological replicate. The agronomic efficiency of N fertilization was calculated as follows: [43, 44]. The collected ripe grains were then used for multi-elemental determination.
Analysis of leaf chlorophyll content and ratio
Chlorophyll content and ratio were measured as a well-established indicator of plant health and nutritional status [45–47]. Fourth leaves collected after root fertilization and flag leaves after foliar treatment were ground in liquid nitrogen and weighed. Total chlorophylls were extracted in buffered 80% aqueous acetone; three technical replicates and three biological replicates were considered for each treatment at each time point. Total chlorophyll content and chla/chlb ratio were determined spectrophotometrically as in [48].
RNA extraction and gene expression analysis
Total RNA was extracted from frozen leaves collected after root and foliar treatments, using the TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA). Purified RNA was submitted to DNase treatment followed by first-strand cDNA synthesis with the SuperScript II Reverse Transcriptase (Thermo Fisher Scientific), according to the product protocol. Real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed for 40 cycles with the Applied Biosystem QuantStudio 3 instrument (Applied Biosystems, Foster City, CA, USA) using Platinum SYBR Green qPCR SuperMix UDG (Thermo Fisher Scientific). Primers for the amplification were designed on the Triticum aestivum genome (IWGSC, INSDC Assembly GCA_900519105.1, Jul 2018; [49]) and are reported in Supplementary Material and Methods. The genes considered were TaNPF5.20 and TaPHT1;6 after root fertilization, as markers for plant N and P status respectively, and TaHAK18, TaACA4 and TaMGT1, to track K, Ca and Mg homeostasis after foliar treatment. Each reaction was performed in triplicate; each of the three pots for each treatment was considered a biological replicate. Melting curve analysis was carried out to ensure the amplification of a single product. Quantitative data were normalized to the endogenous reference genes actin and glyceraldehyde-3-phosphate dehydrogenase, TaGADPH (Supplementary Material and Methods). The 2−ΔΔCT method was used to analyze relative gene expression levels [50]. Primer efficiency was determined to be above 95% using the LinRegPCR v. 2021.2 program [51].
Multi-element determination by energy-dispersive X-ray fluorescence (EDXRF) spectrometry
After harvesting, elemental quantification was performed on ripe grains from Rebelde and Bagou cultivars. UREA, NO3, NO3 + AN and NO3 + VEG treatments were considered for this analysis, with UREA used as a control for the absence of Ca, K and Mg foliar fertilization. The analysis was performed in triplicate, with pots treated as biological replicates. Seeds were dried, finely ground and pressed in 40 mm pellets by a 40-ton pneumatic laboratory press (International Crystal Laboratories, Garfield, NJ, USA) [52]. Multi-element determination was performed by EDXRF spectrometry using an S2 Ranger spectrometer (Bruker, Hamburg, Germany), applying the procedure described in [52].
Statistical analysis
Data in histograms is represented as mean ± standard error. The statistical significance of experimental data was calculated using GraphPad Prism9 (GraphPad Software). Statistical significance was evaluated by a two-way ANOVA followed by a post hoc Tukey’s multiple comparison test. Statistically significant variations (P < 0.05) are marked with letters, the same letter corresponding to statistically non-significant differences. Principal component analysis (PCA) was performed using GraphPad Prism9. Data were standardized automatically before analysis, and principal components were chosen based on the percentage of total explained variance, with a percentage threshold set at 75%.
Results
Effect of root fertilization on wheat growth
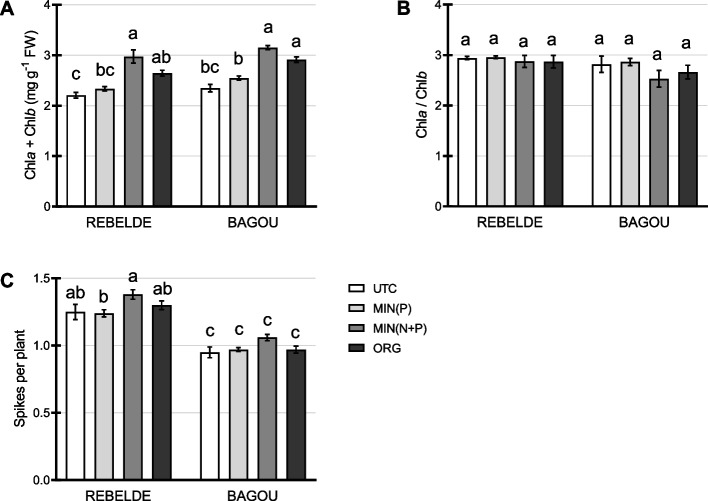
Plant health was evaluated six weeks after root fertilization employing chlorophyll quantification. In both Rebelde and Bagou, MIN(N + P) and ORG treatments showed increased total chlorophyll content, indicative of improved plant health in both cultivars, with a slightly higher increase in the MIN(N + P) treated plants (Fig. 2A): in Rebelde, the increment was of 27% and 14% for the MIN(N + P) and ORG treatments, respectively, compared to MIN(P), whereas in Bagou chlorophyll content reached + 24% and + 14%, respectively. The ratio between chlorophyll a and b was not significantly altered upon fertilization. However, a decrease of 12% was observed upon MIN(N + P) in Bagou (Fig. 2B). The number of productive tillers, measured as the number of spikes per plant, was evaluated at harvesting; only the MIN(N + P) treatment produced a small increase in the mean number of spikes in both genotypes (+ 11% in Rebelde and + 9% in Bagou compared to MIN(P)) (Fig. 2C).
Fig. 2.
Analysis of wheat health and development after root fertilization. Chlorophyll measurements were performed six weeks after root fertilization, on three biological replicates (three pots) for each treatment type; five leaves were pooled from different plants for each biological replicate. The number of productive tillers was evaluated at harvesting on 18 biological replicates. A Total chlorophyll content (biological replicates = 3). B Chlorophyll a/b ratio (biological replicates = 3). C Number of productive tillers, measured as spikes per plant at harvesting (biological replicates = 18). Different letters above the histograms indicate statistical significance, evaluated by two-way ANOVA followed by a post hoc Tukey’s multiple comparison test (P < 0.05)
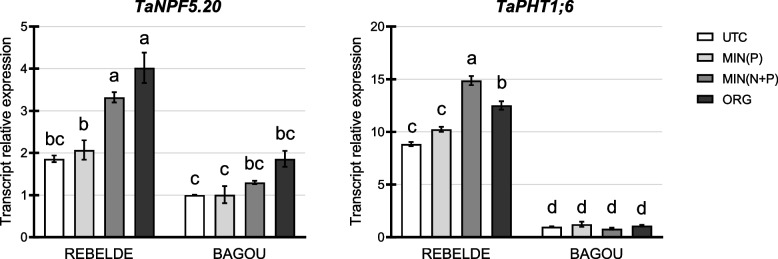
To assess the nutritional status of the plants, expression levels for N transporter NPF5.20 and P transporter PHT1;6 were evaluated by real-time RT-PCR six weeks after root treatment. NPF5.20 is a member of the NPF (Nitrate Transporter 1/Peptide) family, which is involved in nitrate sensing, root absorption and translocation and is up-regulated by N supplementation [53]. In both genotypes of this analysis, transcripts for NPF5.20 were significantly higher in MIN(N + P) and ORG treatments than in UTC and MIN(P) (Fig. 3); in particular, ORG treatment up-regulated NPF5.20 to a higher level, reaching a fold change of about 2 in comparison to the UTC and MIN(P) treatments. As the other marker of plant nutritional status, PHT1;6 is a phosphate transporter induced in both shoots and roots upon P starvation [54, 55]. In this experimental setting, its expression varied only in Rebelde, whereas expression in Bagou was significantly lower and constant in the different treatments. In Rebelde, expression was up-regulated upon combined N and P fertilization in inorganic and organic forms (Fig. 3).
Fig. 3.
Expression analysis of nitrogen and phosphorus transporters after root fertilization. Real-time RT-PCR analysis of TaNPF5.20 and TaPHT1;6, indicative of N and P nutritional status, respectively, measured in leaves six weeks after root fertilization (biological replicates = 3). Different letters above the histograms indicate statistical significance, evaluated by two-way ANOVA followed by a post hoc Tukey’s multiple comparison test (P < 0.05)
Effect of combined root and foliar fertilization on wheat growth
Chlorophyll content was measured one week after foliar treatment, considering each combination of root and foliar fertilization separately. In Rebelde, ANOVA analysis revealed that total chlorophyll concentration was influenced by root fertilization, while foliar fertilization and the interaction between root and foliar fertilization had no significant impact; in particular, MIN(N + P) treatments led to higher chlorophyll levels, with UREA, NO3 and NO3 + VEG foliar fertilization producing significant increases (+ 19%, + 23% and + 21% in comparison to UTC, respectively) (Supplementary Table 1). On the other hand, the chlorophyll a/b ratio was influenced by both foliar and root treatments; however, the impact was only moderately significant, and the pairwise comparison was significant only in the UTC treatment (Supplementary Table 1). In Bagou, both root and foliar fertilization, as well as their interaction, had effects on total chlorophyll content; in particular, the MIN(N + P) treatment had the highest positive impact on chlorophyll content, with the NO3 + AN foliar treatment producing a 44% increase in comparison to UTC and a 17% increase to the MIN(N + P) + UREA treatment (Supplementary Table 1).
Effect of combined root and foliar fertilization on yield parameters
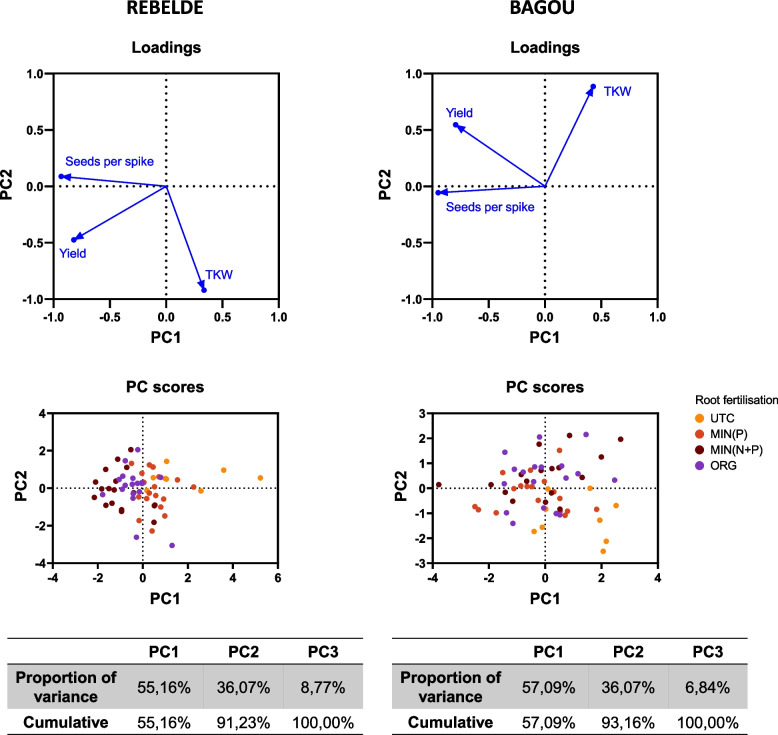
The PCA analysis on wheat yield data evidenced the presence of two synthetic variables explaining more than 90% of the total explained variance (Fig. 4). Based on variable loadings, in both genotypes PC1 significantly influenced the number of seeds per spike and the total yield, whereas PC2 had the highest impact on seed weight; however, in Bagou both variables had a significant effect on yield and seed weight (Fig. 4, Table 3).
Fig. 4.
PCA analysis of yield data after root and foliar fertilization. Left side: wheat cultivar Rebelde; right side: wheat cultivar Bagou. Upper figures: PCA loadings for the three main yield parameters with late onset, i.e., seeds per spike, thousand kernel weight (TKW) and total yield. Middle graphs: sample distribution with respect to the two main principal components (PC1 and PC2); samples are marked with different colors according to their corresponding root treatment. Lower tables: proportion of variance for each principal component
Table 3.
Variable loadings within synthetic variables PC1 and PC2. Highly informative variables (loadings >|0.4|) are highlighted in bold. TKW: thousand kernel weight
| Variable | REBELDE | BAGOU | ||
|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | |
| Seeds per spike | −0.932 | 0.087 | −0.947 | −0.057 |
| TKW | 0.335 | −0.921 | 0.428 | 0.884 |
| Yield | −0.820 | −0.475 | −0.794 | 0.545 |
In Rebelde, the yield was significantly increased upon fertilization (Fig. 5) and influenced by both root and foliar treatments; in particular, the yield parameter was a result of the combination of spike number and seeds per spike, both influenced by root fertilization, and the seed weight, mainly associated with foliar fertilization (Fig. 2, Supplementary Table 1). Overall, the MIN(N + P) root treatment had the most beneficial effect on yield by acting on the number of spikes per plant and seeds per spike; moreover, this root treatment shows the most evident effects upon foliar spraying. Notably, three subsets from the MIN(N + P) treatment had significantly higher yields in comparison to UTC and MIN(P) plants: UREA + VEG (+ 49% to UTC), NO3 (+ 50% to UTC) and NO3 + VEG (+ 55% to UTC); the VEG biostimulant produced the most positive effect on grain yield (Fig. 5). Regarding the contribution of foliar fertilization on yield components, the NO3 treatments (with or without biostimulants) provided the highest increase in TKW across all root amendments (Supplementary Table 1). In the MIN(N + P) background, this generates an increment in yield of + 13%, + 10% and + 17% in the NO3, NO3 + AN and NO3 + VEG foliar treatments in comparison to UREA, respectively; similarly, the yield increase was of + 9%, + 15% and + 14% in the ORG background (Fig. 5, Supplementary Table 1). In Bagou, yield parameters were mostly stable upon the different treatments; no statistically significant influence of either root or foliar fertilization was identified by two-way ANOVA analysis (Fig. 5, Supplementary Table 1).
Fig. 5.
Analysis of wheat yield after root and foliar fertilization. Yield per hectare in Rebelde and Bagou, measured at harvesting of ripe heads (biological replicates = 3). Different letters above the histograms indicate statistical significance, evaluated by two-way ANOVA followed by a post hoc Tukey’s multiple comparison test (P < 0.05)
Consistent with yield results, agronomic efficiency (i.e., the efficiency of N used to produce increased yield) in Rebelde was influenced by both root and foliar fertilization, as highlighted by the two-way ANOVA results. In particular, the NO3 treatment had an overall increased efficiency of N use across all three root strategies; agronomic efficiency was especially high upon the MIN(P) treatment, where the lack of root N fertilization leads to more effective uptake of foliar N (Supplementary Table 1). On the other hand, the response of Bagou wheat to the different treatments was not well defined; however, ANOVA analysis identified root fertilization as accounting significantly for variation. As in Rebelde, agronomic efficiency was particularly high in some foliar treatments associated with the MIN(P) root fertilization, although statistical significance was generally poor (Supplementary Table 1).
Effect of foliar fertilization on the mineral composition of wheat grain
Foliar fertilization with Ca, K and Mg nitrates had limited effects on the content of these elements in wheat grain, as measured in ripe grains after harvesting. A moderate increase was observed for Ca in Rebelde, although multiple comparison tests identified no significance; similarly, Mg content was increased by NO3 fertilization in Bagou (Supplementary Table 2). However, the two-way ANOVA analysis of elemental composition evidenced a different behavior of the two genotypes in response to the applied treatments. Rebelde was more sensitive to root fertilization, which affected Ca, Cl, K, Mg, sulfur (S) and P. In contrast, only Ca, Cl and S variations were associated with foliar fertilization (Fig. 6, Supplementary Table 2). On the other hand, variations in Bagou were driven mainly by foliar treatment, which affected Cl, Mg, S and P content in grains (Fig. 6, Supplementary Table 2). Overall, despite the limited results produced by nitrate fertilization on Ca, K and Mg grain content, this foliar treatment had a significant effect in reducing Cl content, as well as increasing S accumulation in grains; no specific effect was observed upon biostimulant application (Fig. 6, Supplementary Table 2).
Fig. 6.
Elemental composition of wheat grain upon foliar fertilization. Concentrations of the most significant macronutrients in Rebelde (left) and Bagou (right) grain, measured on ripe grains after harvesting by energy-dispersive X-ray fluorescence (EDXRF) spectrometry (n = 3). A Ca, B K, C Mg, D Cl, E S, F P. Different letters above the histograms indicate statistical significance, evaluated by two-way ANOVA followed by a post hoc Tukey’s multiple comparison test (P < 0.05)
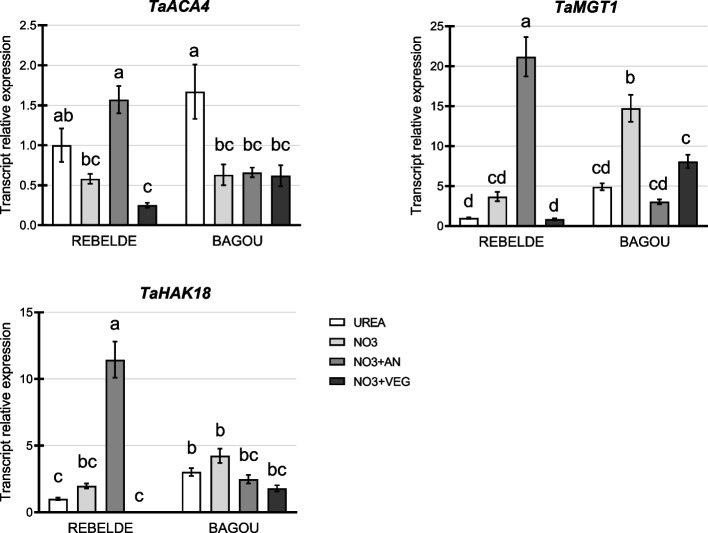
The expression of cation transporters ACA4, MGT1 and HAK18 was measured in the MIN(N + P) root background to estimate Ca, Mg and K nutritional status upon foliar treatment; the MIN(N + P) fertilization was chosen as the one showing overall the biggest differences in the parameters considered till now. ACA4, a tonoplast ATPase induced upon Ca stress [56], was down-regulated upon Ca supplementation in both genotypes, with the exclusion of the NO3 + AN treatment in Rebelde (Fig. 7). Expression of MGT1 and HAK18, Mg and K transporters, respectively [57, 58], has similar profiles in both genotypes. Their expression in Rebelde is low in all treatments apart from NO3 + AN; conversely, transcription in Bagou is higher in the NO3 foliar application, although the difference is statistically significant only for MGT1 (Fig. 7).
Fig. 7.
Expression analysis of cation transporters after foliar fertilization. Real-time RT-PCR analysis of TaACA4, TaMGT1 and TaHAK18, considered for Ca, Mg and K respectively, measured one week after foliar fertilization in flag leaves (biological replicates = 3). The analysis was performed in the MIN(N + P) root fertilization background as the one showing the biggest differences between foliar treatments when considering the health and yield parameters. Different letters above the histograms indicate statistical significance, evaluated by two-way ANOVA followed by a post hoc Tukey’s multiple comparison test (P < 0.05)
Discussion
Fertilization is a core point in recent agricultural research. Novel fertilization strategies aim to effectively improve plant growth, yield, and food quality while promoting economic and environmental sustainability [16, 19]. In this context, organic amendments and biostimulants are emerging as viable alternatives to conventional chemical fertilization [16, 22]. This study investigates the application of alternative fertilization methods at a microcosm scale in wheat, seeking more sustainable approaches for plant nutrition, yield, and grain quality.
The split fertilization regime, splitting or delaying the fertilizer application across different stages of plant life to meet the crop’s demand, has been widely applied in wheat cultivation and is supported by scientific evidence that recognizes its effect of increasing yield and/or grain quality [59, 60]. Among the available strategies, root fertilization has been recognized as the best N supplementation method, whereas foliar treatments have been recommended for corrective and/or boosting approaches [31, 32]. The application of foliar fertilization in the heading stage allows the easy delivery of readily available nutrients, including cationic nutrients, during grain development and filling [32]. Furthermore, this method aligns well with agricultural practices in the Italian Po plain, where it can be combined with pest management strategies required in late spring in this area.
Root and foliar fertilization contribute differently to plant health and productivity
Consistent with existing literature [31, 32], root treatment emerged as the primary determinant of plant health and productivity differences in both cultivars considered in this work. Indeed, it influenced chlorophyll content, the number of spikes in both Rebelde and Bagou, the number of seeds per spike, and the global yield in Rebelde. On the other hand, foliar spraying impacted chlorophyll content in Bagou, in combination with root supplementation, and modulated seed weight in Rebelde. The effect of foliar fertilization on yield is still a debated issue. Although evidence suggests that it can be as effective as basal nutrition [30], its impact is higher when combined with root fertilization. It can be significantly modulated by the dosage of the latter [61, 62]. As for this study, the more significant impact of root fertilization can likely be ascribed to the higher dosage and application timing. Indeed, in this case, root and foliar supplementation act on different yield components, which are generated at various moments of the wheat developmental timeline [63]. In Rebelde, where treatment contribution was more evident, root fertilization brought changes to yield components that have an early origin, i.e., the number of spikes and seeds per spike; conversely, foliar treatment played a crucial role in determining a late component, seed weight, with nitrate application and vegetal biostimulant producing the best outcomes. Late-season foliar fertilization, in particular with urea or nitrates, has been proven effective in boosting grain filling [64, 65]; also, the application of biostimulants, e.g., hydrolyzed extracts from different sources, has been reported to increase plant yield [21–24, 27].
Organic root fertilization is effective in improving grain yield
The application of organic products in wheat nutrition has been widely discussed, in view of increasing the sustainability of the agricultural process, although most experiments rely on a partial substitution of chemical fertilization [66–69]. In this work, the most significant effects on plant health and yield were achieved with the mineral root treatment, confirming that chemical fertilization is more readily available and more efficient in boosting plant growth than organic [70, 71]. On the other hand, organic root treatment also produced comparable results when considering chlorophyll content and yield. Notably, the expression of the nitrate transporter NPF5.20, reported as induced by N supplementation [53], was higher in organic fertilization in both genotypes, suggesting that a greater N supply is still available in the soil upon this condition after six weeks. Together with the results achieved for chlorophyll content and spike number, this is consistent with the slower release of N in organic fertilizers, indicating that organic amendments can contribute beneficially to long-term agricultural strategies [72].
Fertilization efficacy depends on the genotype cultivated
Our results highlighted that different cultivars or genotypes may display distinct nutrient requirements, which must be considered when planning fertilization. Indeed, yield components in Rebelde were significantly modulated by fertilization, whereas yield in Bagou remained largely unaffected by either fertilization type. This is coherent with previous evidence demonstrating different nutritional needs in different cultivars, and it has been proposed that yield and grain protein content are the major determinants of N requirements, together with other components of genetically determined N use efficiency [73–75]. Under this point of view, Bagou may be considered a low-N cultivar, thus likely requiring lower levels of N than Rebelde to achieve comparable yields. Also, P content in Bagou seeds is markedly lower. Consistent with this, yield increases upon fertilization are non-significant in Bagou while reaching about + 50% in Rebelde. This hypothesis is also supported by the transcript levels of N and P transporters upon root fertilization, which are significantly lower in Bagou. In particular, the expression of P transporter PHT1;6, usually induced upon P deficiency [54, 55], was especially low in Bagou and did not respond to nutrient supplementation, suggesting that Bagou was already in a P-sufficient condition in the untreated control. On the contrary, PHT1;6 in Rebelde was responsive to N supplementation. Although the response of wheat P transporters to N availability has not yet been reported in the literature, evidence suggests that N and P homeostasis are interconnected, and N level and form can influence P uptake [76]. In this view, Rebelde’s higher N requirements may also reflect on its management of P nutrition. Overall, these data support the necessity of considering genotype specificity to tailor the fertilization strategies according to their needs.
Foliar treatment modulates the grain ionome
In this experiment, foliar treatments also aimed to enhance the nutritional quality of grains by increasing the content of cation nutrients Ca, Mg, and K. Overall, nutrient content was more stable in Bagou. Still, it showed greater variability in Rebelde across both root and foliar treatments. Biofortification with cations was only partially successful in both wheat cultivars. A statistically significant increase (up to + 15% compared to foliar urea) was achieved for Ca in Rebelde, enhanced by biostimulants, and Mg in Bagou. Despite being modest, the results obtained in biofortification were comparable with previous observations that highlighted the dependence on the cultivar considered [40]. Analogously, although biostimulants were proposed to promote nutrient uptake through multiple mechanisms [21, 22, 77], only minimal changes in Ca, Mg and K were reported in wheat when combining biostimulants with macro- and micronutrient formulations [23, 27, 78]. The expression profiles of cation transporters observed in this work reflected the nutrient levels accumulated in grains. For instance, ACA4 was proposed as induced upon Ca stress, both as deficiency and excess [56], and indeed, Ca supplementation down-regulates ACA4 expression in this experiment, indicating a likely alleviation of Ca deficiency. On the other hand, both MGT1 and HAK18 are not consistently modulated in the different treatments, reflecting negligible changes in Mg and K content following foliar applications. MGT1 is an Mg transporter that was proposed to be down-regulated during long-term Mg deficiency [57]; HAK18 is a high-affinity K transporter that was reported to be up-regulated upon K deficiency [58]. The choice of marker genes, which may be correlated to micronutrient status, is a limitation of this approach. In wheat, these genes are part of large families and, despite recent research, have still poorly characterized tissue specificity and expression profile, thus making the choice of an adequate marker difficult. Moreover, most findings were derived from growth protocols that do not mimic field conditions [56–58], and, therefore, are difficult to extend to more complex environmental conditions. Despite these limitations, all three transporters were significantly upregulated in the nitrate + animal biostimulant foliar treatment. Biostimulants have generally been shown to induce modulation of gene expression. Specifically, there is evidence for the effects of animal hydrolysates on the transcript levels of genes involved in nutrient transport [79, 80].
Interestingly, further changes were observed in the grain ionome upon foliar treatment. Nitrate application significantly decreased Cl and increased S in both cultivars and P levels in Bagou. In these cases, biostimulants had a mixed effect on elemental composition. Both Cl and S play an important role in grain quality. Low-S grains have been associated with altered amino acid and protein composition, impaired baking properties, and reduced nutritional quality, since S-containing amino acids are essential for the human diet [81, 82]. On the contrary, Cl plays a more controversial role in crops: it is crucial for plant nutrition [83–85] and enhances wheat flour properties [86], but excessive Cl can hinder plant growth and yield [83, 85]. Cl is an essential nutrient in the human diet, but its requirements are difficult to define since Cl is assumed chiefly in combination with sodium (Na) [87]; however, the differences observed in Cl content are not enough to raise health concerns. It is interesting to note that these ionome changes were detected even in front of an equal S and P supplementation. In this view, it should be remembered that all these homeostatic pathways are strictly interconnected; for example, N nutritional status strongly influences and is at the same time modulated by S and P assimilation, and NPK fertilization has been shown to affect grain S content in relation to protein quality [88–91]. Additionally, Cl competes with nitrate ions for uptake and transport [83, 85, 92], which explains the reduction observed with nitrate foliar fertilization.
Conclusion
This work proposes alternative strategies that have proved similarly effective to chemical fertilization. Root fertilization was found to be the primary driver of plant health and yield, although the contribution of foliar treatment was not insignificant. Regarding the former, organic root fertilization, considered in view of sustainable agricultural waste recycling, was validated as an effective alternative to mineral application, producing comparable yield while offering extended nutrient availability. Moreover, foliar treatment with nitrates, also combined with biostimulants from plant and animal residuals, positively influenced the elemental composition of wheat grains. However, the supplementation at heading with cation nutrients Ca, Mg and K led to minimal results and could not produce meaningful biofortification in wheat grain. Most importantly, the genotype-specific effect of the different fertilization strategies on yield and global nutrient assimilation is significant. This evidence underscores the necessity of conducting preliminary assessments of specific nutritional needs to optimize results across different cultivars.
Supplementary Information
Supplementary Material 1: Supplementary Material and Methods. Composition of growth substrate, mineral and organic fertilization sources and biostimulants. List of primers used for real-time RT-PCR.
Supplementary Material 2: Supplementary Table 1. Health and yield parameters in Rebelde and Bagou upon foliar fertilization.
Supplementary Material 3: Supplementary Table 2. Elemental composition of Rebelde and Bagou grains, measured by EDXRF.
Acknowledgements
Not applicable.
Authors’ contributions
Conceptualization: EF, CF, AF and GDC. Experiment conduction, result analysis and data presentation: EF, CF and GDC. Writing: EF. Writing—review and editing: CF, AF and GDC. Funding acquisition: AF and GDC. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the European Social Fund (project code 1695-0002-1463-2019).
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Antonella Furini, Email: antonella.furini@univr.it.
Giovanni DalCorso, Email: giovanni.dalcorso@univr.it.
References
- 1.Carew R, Smith EG, Grant C. Factors influencing wheat yield and variability: evidence from Manitoba, Canada. J Agric Appl Econ. 2009;41(3):625–39. [Google Scholar]
- 2.Anderson WK. Closing the gap between actual and potential yield of rainfed wheat. The impacts of environment, management and cultivar. Field Crops Res. 2010;116:14–22. [Google Scholar]
- 3.Bakker MM, Govers G, Ewert F, Rounsevell M, Jones R. Variability in regional wheat yields as a function of climate, soil and economic variables: assessing the risk of confounding. Agric Ecosyst Environ. 2005;110(3–4):195–209. [Google Scholar]
- 4.Krupnik TJ, Ahmed ZU, Timsina J, Yasmin S, Hossain F, Al Mamun A, et al. Untangling crop management and environmental influences on wheat yield variability in Bangladesh: an application of non-parametric approaches. Agric Syst. 2015;139:166–79. [Google Scholar]
- 5.Vogel KP, Johnson VA, Mattern PJ. Protein and lysine content of grain, endosperm, and bran of wheats from the USDA world wheat collection. Crop Sci. 1976;16(5):655–60. [Google Scholar]
- 6.Rakszegi M, Boros D, Kuti C, Láng L, Bedo Z, Shewry PR. Composition and end-use quality of 150 wheat lines selected for the HEALTHGRAIN diversity screen. J Agric Food Chem. 2008;56(21):9750–7. [DOI] [PubMed] [Google Scholar]
- 7.Battenfield SD, Guzmán C, Gaynor RC, Singh RP, Peña RJ, Dreisigacker S, Fritz AK, Poland JA. Genomic Selection for Processing and End-Use Quality Traits in the CIMMYT Spring Bread Wheat Breeding Program. Plant Genome. 2016;9(2). 10.3835/plantgenome2016.01.0005. [DOI] [PubMed]
- 8.Guzman C, Peña RJ, Singh R, Autrique E, Dreisigacker S, Crossa J, et al. Wheat quality improvement at CIMMYT and the use of genomic selection on it. Appl Transl Genomics. 2016;11:3–8. Elsevier B.V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benini M, Blasi E, Detti P, Fosci L. Solving crop planning and rotation problems in a sustainable agriculture perspective. Comput Oper Res. 2023;1:159. [Google Scholar]
- 10.Chuan L, He P, Jin J, Li S, Grant C, Xu X, et al. Estimating nutrient uptake requirements for wheat in China. Field Crops Res. 2013;146:96–104. [Google Scholar]
- 11.Pathak H, Aggarwal PK, Roetter R, Kalra N, Bandyopadhaya SK, Prasad S, et al. Modelling the quantitative evaluation of soil nutrient supply, nutrient use efficiency, and fertilizer requirements of wheat in India. Nutr Cycl Agroecosyst. 2003;65:105. [Google Scholar]
- 12.Malik AH, Kuktaite R, Johansson E. Combined effect of genetic and environmental factors on the accumulation of proteins in the wheat grain and their relationship to bread-making quality. J Cereal Sci. 2013;57(2):170–4. [Google Scholar]
- 13.Horvat D, Loncaric Z, Vukadinovic V, Drezner G, Bertic B, Dvojkovic K. The influence of mineral fertilization on winter wheat yield and quality. Cereal Res Commun. 2006;34(1):429–32. [Google Scholar]
- 14.Zecevic V, Knezevic D, Boskovic J, Micanovic D, Dozet G. Effect of nitrogen fertilization on winter wheat quality. Cereal Res Commun. 2010;38(2):243–9. [Google Scholar]
- 15.Montemurro F, Convertini G, Ferri D. Nitrogen application in winter wheat grown in Mediterranean conditions: effects on nitrogen uptake, utilization efficiency, and soil nitrogen deficit. J Plant Nutr. 2007;30(10):1681–703. [Google Scholar]
- 16.Tyagi J, Ahmad S, Malik M. Nitrogenous fertilizers: impact on environment sustainability, mitigation strategies, and challenges. Int J Environ Sci Technol. 2022;19:11649–72. [Google Scholar]
- 17.Udeigwe TK, Teboh JM, Eze PN, Hashem Stietiya M, Kumar V, Hendrix J, et al. Implications of leading crop production practices on environmental quality and human health. J Environ Manag. 2015;151:267–79. Academic Press. [DOI] [PubMed] [Google Scholar]
- 18.Ugulu I, Ahmad K, Khan ZI, Munir M, Wajid K, Bashir H. Effects of organic and chemical fertilizers on the growth, heavy metal/metalloid accumulation, and human health risk of wheat (Triticum aestivum L.). EnvironSci Pollut Res. 2021;28:12533–45. [DOI] [PubMed] [Google Scholar]
- 19.Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–7. [DOI] [PubMed] [Google Scholar]
- 20.Folina A, Tataridas A, Mavroeidis A, Kousta A, Katsenios N, Efthimiadou A, Travlos IS, Roussis I, Darawsheh MK, Papastylianou P, et al. Evaluation of various nitrogen indices in N-Fertilizers with inhibitors in field crops: a review. Agronomy. 2021;11:418. [Google Scholar]
- 21.Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH. Biostimulants in plant science: a global perspective. Front Plant Sci. 2017;7:2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvo P, Nelson L, Kloepper JW. Agricultural uses of plant biostimulants. Plant Soil. 2014;383(1–2):3–41. [Google Scholar]
- 23.Maignan V, Géliot P, Avice JC. Glutacetine® biostimulant applied on wheat under contrasting field conditions improves grain number leading to better yield, upgrades n-related traits and changes grain ionome. Plants. 2021;10(3):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pačuta V, Rašovský M, Michalska-Klimczak B, Wyszyňski Z. Grain yield and quality traits of durum wheat (Triticum durum desf.) treated with seaweed-and humic acid-based biostimulants. Agronomy. 2021;11(7):1270. [Google Scholar]
- 25.Wang S, Tian X, Liu Q. The effectiveness of foliar applications of zinc and biostimulants to increase zinc concentration and bioavailability of wheat grain. Agron. 2020;10(2):178. 10.3390/agronomy10020178.
- 26.Radzikowska-Kujawska D, John P, Piechota T, Nowicki M, Kowalczewski PŁ. Response of winter wheat (Triticum aestivum L.) to selected biostimulants under drought conditions. Agriculture. 2023;13(1):121. [Google Scholar]
- 27.Popko M, Michalak I, Wilk R, Gramza M, Chojnacka K, Górecki H. Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules. 2018;23(2):470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu L, Geelen D. Developing Biostimulants from agro-food and industrial by-products. Front Plant Sci. 2018;9:1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dass A, Rajanna GA, Babu S, Lal SK, Choudhary AK, Singh R, et al. Foliar application of macro-and micronutrients improves the productivity, economic returns, and resource-use efficiency of soybean in a semiarid climate. Sustainability. 2022;14(10):5825. [Google Scholar]
- 30.Ferrari M, Dal Cortivo C, Panozzo A, Barion G, Visioli G, Giannelli G, et al. Comparing soil vs. foliar nitrogen supply of the whole fertilizer dose in common wheat. Agronomy. 2021;11(11):2138. [Google Scholar]
- 31.Niu J, Liu C, Huang M, Liu K, Yan D. Effects of foliar fertilization: a review of current status and future perspectives. J Soil Sci Plant Nutr. 2021;21:104–18. [Google Scholar]
- 32.Fageria NK, Filho MPB, Moreira A, Guimarães CM. Foliar fertilization of crop plants. J Plant Nutr. 2009;32(6):1044–64. [Google Scholar]
- 33.Fernandez V, Eichert T. Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. CRC Crit Rev Plant Sci. 2009;28(1–2):36–68. [Google Scholar]
- 34.Gupta PK, Balyan HS, Sharma S, Kumar R. Biofortification and bioavailability of Zn, Fe and Se in wheat: present status and future prospects. Theor Appl Genet. 2021;134:1–35. [DOI] [PubMed] [Google Scholar]
- 35.Velu G, Ortiz-Monasterio I, Cakmak I, Hao Y, Singh RP. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J Cereal Sci. 2014;59:365e372. [Google Scholar]
- 36.Szerement J, Szatanik-Kloc A, Mokrzycki J, Mierzwa-Hersztek M. Agronomic biofortification with Se, Zn, and Fe: an effective strategy to enhance crop nutritional quality and stress defense—a review. J Soil Sci Plant Nutr. 2022;22:1129–59. [Google Scholar]
- 37.Broadley MR, White PJ. Eats roots and leaves. Can edible horticultural crops address dietary calcium, magnesium and potassium deficiencies? Proc Nutr Soc. 2010;69(4):601–12. [DOI] [PubMed] [Google Scholar]
- 38.Osuna-Padilla IA, Leal-Escobar G, Garza-García CA, Rodríguez-Castellanos FE. Carga ácida de la dieta; mecanismos y evidencia de sus repercusiones en la salud. Nefrologia. 2019;39(4):343–54. [DOI] [PubMed] [Google Scholar]
- 39.Wieërs MLAJ, Beynon-Cobb B, Visser WJ, Attaye I. Dietary acid load in health and disease. Pflugers Arch. 2024;476(4):427–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cobalchin F, Volpato M, Modena A, Finotti L, Manni F, Panozzo A, et al. Biofortification of common wheat grains with combined Ca, Mg, and K through foliar fertilisation. Agronomy. 2021;11(9):1718. [Google Scholar]
- 41.Blandino M, Marinaccio F, Reyneri A. Effect of late-season nitrogen fertilization on grain yield and on flour rheological quality and stability in common wheat, under different production situations. Ital J Agron. 2016;11(2):107–13. [Google Scholar]
- 42.Blandino M, Marinaccio F, Vaccino P, Reyneri A. Nitrogen fertilization strategies suitable to achieve the quality requirements of wheat for biscuit production. Agron J. 2015;107(4):1584–94. [Google Scholar]
- 43.Dobermann AR. Nitrogen Use Efficiency – State of the Art. Agronomy & Horticulture -- Faculty Publications. 2005;316. https://digitalcommons.unl.edu/agronomyfacpub/316.
- 44.Congreves KA, Otchere O, Ferland D, Farzadfar S, Williams S, Arcand MM. Nitrogen use efficiency definitions of today and tomorrow. Front Plant Sci. 2021;12:637108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlovic D, Nikolic B, Djurovic S, Waisi H, Andjelkovic A, Marisavljevic D. Chlorophyll as a measure of plant health: agroecological aspects. Pestic Phytomed. 2014;29(1):21–34. [Google Scholar]
- 46.Shah SH, Houborg R, McCabe MF. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.). Agronomy. 2017;7:61. [Google Scholar]
- 47.Zhang H, Ge Y, Xie X, Atefi A, Wijewardane NK, Thapa S. High throughput analysis of leaf chlorophyll content in sorghum using RGB, hyperspectral, and fluorescence imaging and sensor fusion. Plant Methods. 2022;18(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porra RJ. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res. 2002;73:149–56. [DOI] [PubMed] [Google Scholar]
- 49.Appels R, Eversole K, Feuillet C, Keller B, Rogers J, Stein N, et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. 1979;2018(361):6403. [DOI] [PubMed] [Google Scholar]
- 50.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 51.Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJB, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37(6):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berzaghi P, Lotto A, Mancinelli M, Benozzo F. Technical note: Rapid mineral determination in forages by X-ray fluorescence. J Dairy Sci. 2018;101(11):9967–70. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Wan Y, Buchner P, King R, Ma H, Hawkesford MJ. Phylogeny and gene expression of the complete nitrate transporter 1/peptide transporter family in triticum aestivum. J Exp Bot. 2020;71(15):4531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teng W, Zhao YY, Zhao XQ, He X, Ma WY, Deng Y, et al. Genome-wide identification, characterization, and expression analysis of PHT1 phosphate transporters in wheat. Front Plant Sci. 2017;8:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grün A, Buchner P, Broadley MR, Hawkesford MJ. Identification and expression profiling of Pht1 phosphate transporters in wheat in controlled environments and in the field. Plant Biol. 2018;20(2):374–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aslam R, Williams LE, Bhatti MF, Virk N. Genome-wide analysis of wheat calcium ATPases and potential role of selected ACAs and ECAs in calcium stress. BMC Plant Biol. 2017;17(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang Y, Yang X, Li H, Shuai Y, Chen W, Ma D, et al. Uncovering the role of wheat magnesium transporter family genes in abiotic responses. Front Plant Sci. 2023;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng X, Liu X, Mao W, Zhang X, Chen S, Zhan K, et al. Genome-wide identification and analysis of HAK/KUP/KT potassium transporters gene family in wheat (Triticum aestivum L.). Int J Mol Sci. 2018;19(12):3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu C, Sadras VO, Lu G, Zhang P, Han Y, Liu L, et al. A global meta-analysis of split nitrogen application for improved wheat yield and grain protein content. Soil Tillage Res. 2021;213:105111. [Google Scholar]
- 60.Wu W, Wang Y, Wang L, Xu H, Zörb C, Geilfus CM, et al. Booting stage is the key timing for split nitrogen application in improving grain yield and quality of wheat – A global meta-analysis. Field Crops Res. 2022;287:108665. [Google Scholar]
- 61.Hang Z, Yu Z, Zhang Y, Shi Y. Optimized nitrogen fertilizer application strategies under supplementary irrigation improved winter wheat (Triticumaestivum L.) yield and grain protein yield. PeerJ. 2021;9:e11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varga B, Svečnjak Z. The effect of late-season urea spraying on grain yield and quality of winter wheat cultivars under low and high basal nitrogen fertilization. Field Crops Res. 2006;96(1):125–32. [Google Scholar]
- 63.Slafer GA, Savin R, Sadras VO. Coarse and fine regulation of wheat yield components in response to genotype and environment. Field Crops Res. 2014;5(157):71–83. [Google Scholar]
- 64.Lv X, Ding Y, Long M, Liang W, Gu X, Liu Y, et al. Effect of foliar application of various nitrogen forms on starch accumulation and grain filling of wheat (Triticum aestivum L.) under drought stress. Front Plant Sci. 2021;12:645379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyu X, Liu Y, Li N, Ku L, Hou Y, Wen X. Foliar applications of various nitrogen (N) forms to winter wheat affect grain protein accumulation and quality via N metabolism and remobilization. Crop Journal. 2022;10(4):1165–77. [Google Scholar]
- 66.He H, Peng M, Lu W, Hou Z, Li J. Commercial organic fertilizer substitution increases wheat yield by improving soil quality. Sci Total Environ. 2022;10(851):158132. [DOI] [PubMed] [Google Scholar]
- 67.Tosti G, Farneselli M, Benincasa P, Guiducci M. Nitrogen fertilization strategies for organic wheat production: crop yield and nitrate leaching. Agron J. 2016;108(2):770–81. [Google Scholar]
- 68.Wang L, Li Q, Coulter JA, Xie J, Luo Z, Zhang R, et al. Winter wheat yield and water use efficiency response to organic fertilization in northern China: a meta-analysis. Agric Water Manag. 2020;28:229. [Google Scholar]
- 69.Kubar MS, Zhang Q, Feng M, Wang C, Yang W, Kubar KA, et al. Growth, yield and photosynthetic performance of winter wheat as affected by co-application of nitrogen fertilizer and organic manures. Life. 2022;12(7):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Niedziński T, Sierra MJ, Łabętowicz J, Noras K, Cabrales C, Millán R. Release of nitrogen from granulate mineral and organic fertilizers and its effect on selected chemical parameters of soil. Agronomy. 2021;11(10):1981. [Google Scholar]
- 71.Xin X, Qin S, Zhang J, Zhu A, Yang W, Zhang X. Yield, phosphorus use efficiency and balance response to substituting long-term chemical fertilizer use with organic manure in a wheat-maize system. Field Crops Res. 2017;208:27–33. [Google Scholar]
- 72.Yang YJ, Lei T, Du W, Liang CL, Li HD, Lv JL. Substituting chemical fertilizer nitrogen with organic manure and comparing their nitrogen use efficiency and winter wheat yield. J Agric Sci. 2020;158(4):262–8. [Google Scholar]
- 73.Le Gouis J, Béghin D, Heumez E, Pluchard P. Genetic differences for nitrogen uptake and nitrogen utilisation efficiencies in winter wheat. Eur J Agron. 2000;3–4:163–73. [Google Scholar]
- 74.Hawkesford MJ. The diversity of nitrogen use efficiency for wheat varieties and the potential for crop improvement. Better Crops. 2012;96(3):10–2. [Google Scholar]
- 75.Saleem MF, Ma BL, Voldeng H, Wang TC. Nitrogen nutrition on leaf chlorophyll, canopy reflectance, grain protein and grain yield of wheat varieties with contrasting grain protein concentration. J Plant Nutr. 2010;33(11):1681–95. [Google Scholar]
- 76.Zhang F, Kang S, Zhang J, Zhang R, Li F. Nitrogen fertilization on uptake of soil inorganic phosphorus fractions in the wheat root zone. Soil Sci Soc Am J. 2004;68(6):1890–5. [Google Scholar]
- 77.Halpern M, Bar-Tal A, Ofek M, Minz D, Muller T, Yermiyahu U. The use of biostimulants for enhancing nutrient uptake. Adv Agron. 2015;130:141–74. [Google Scholar]
- 78.Maignan V, Bernay B, Géliot P, Avice JC. Biostimulant effects of Glutacetine® and its derived formulations mixed with N Fertilizer on post-heading n uptake and remobilization, seed yield, and grain quality in winter wheat. Front Plant Sci. 2020;13(11):607615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trevisan S, Manoli A, Ravazzolo L, Franceschi C, Quaggiotti S. mRNA-sequencing analysis reveals transcriptional changes in root of maize seedlings treated with two increasing concentrations of a new biostimulant. J Agric Food Chem. 2017;65(46):9956–69. [DOI] [PubMed] [Google Scholar]
- 80.Santi C, Zamboni A, Varanini Z, Pandolfini T. Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front Plant Sci. 2017;30(8):433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao FJ, Salmon SE, Withers PJA, Evans EJ, Monaghan JM, Shewry PR, et al. Responses of breadmaking quality to sulphur in three wheat varieties. J Sci Food Agric. 1999;79(13):1865–74. [Google Scholar]
- 82.Wrigley CW, Du Cros DL, Archer MJ, Downie PG, Roxburgh CM. The sulfur content of wheat endosperm proteins and its relevance to grain quality. Aust J Plant Physiol. 1980;7:755–66. [Google Scholar]
- 83.Geilfus CM. Chloride: from nutrient to toxicant. Plant Cell Physiol. 2018;59(5):877–86. [DOI] [PubMed] [Google Scholar]
- 84.Colmenero-Flores JM, Franco-Navarro JD, Cubero-Font P, Peinado-Torrubia P, Rosales MA. Chloride as a beneficial macronutrient in higher plants: new roles and regulation. Int J Mol Sci. 2019;20(19):4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Geilfus CM. Review on the significance of chlorine for crop yield and quality. Plant Sci. 2018;270:114–22. Elsevier Ireland Ltd. [DOI] [PubMed] [Google Scholar]
- 86.Bosmans GM, Peene LJ, Van Haesendonck I, Brijs K, Delcour JA. Impact of chlorine treatment on properties of wheat flour and its components in the presence of sucrose. Food Chem. 2019;274:434–43. [DOI] [PubMed] [Google Scholar]
- 87.Strohm D, Bechthold A, Ellinger S, Leschik-Bonnet E, Stehle P, Heseker H. Revised reference values for the intake of sodium and chloride. Ann Nutr Metab. 2018;72(1):12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DuPont FM, Hurkman WJ, Vensel WH, Chan R, Lopez R, Tanaka CK, et al. Differential accumulation of sulfur-rich and sulfur-poor wheat flour proteins is affected by temperature and mineral nutrition during grain development. J Cereal Sci. 2006;44(1):101–12. [Google Scholar]
- 89.Courbet G, Gallardo K, Vigani G, Brunel-Muguet S, Trouverie J, Salon C, et al. Disentangling the complexity and diversity of crosstalk between sulfur and other mineral nutrients in cultivated plants. J Exp Bot. 2019;70:4183–96. Oxford University Press. [DOI] [PubMed] [Google Scholar]
- 90.Duncan EG, O’Sullivan CA, Roper MM, Biggs JS, Peoples MB. Influence of co-application of nitrogen with phosphorus, potassium and sulphur on the apparent efficiency of nitrogen fertiliser use, grain yield and protein content of wheat: review. Field Crop Res. 2018;226:56–65. [Google Scholar]
- 91.Krouk G, Kiba T. Nitrogen and Phosphorus interactions in plants: from agronomic to physiological and molecular insights. Curr Opin Plant Biol. 2020;57:104–9. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- 92.Zhang X, Franzisky BL, Eigner L, Geilfus CM, Zörb C. Antagonism of chloride and nitrate inhibits nitrate reductase activity in chloride-stressed maize. Plant Growth Regul. 2021;93(3):279–89. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplementary Material and Methods. Composition of growth substrate, mineral and organic fertilization sources and biostimulants. List of primers used for real-time RT-PCR.
Supplementary Material 2: Supplementary Table 1. Health and yield parameters in Rebelde and Bagou upon foliar fertilization.
Supplementary Material 3: Supplementary Table 2. Elemental composition of Rebelde and Bagou grains, measured by EDXRF.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.