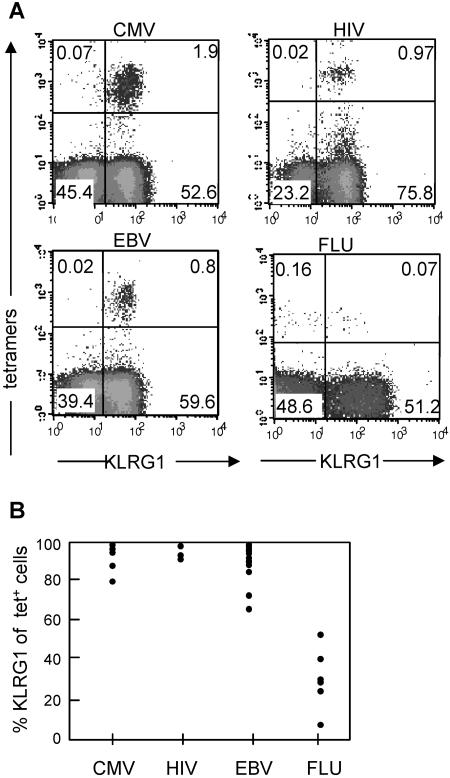
FIG. 3.
Expression of KLRG1 by virus-specific CD8 T cells in humans. (A) PBLs from donors that were known to possess traceable numbers of virus-specific T cells were stained by the indicated tetramers (A2/pp65 for CMV, B8/Nef for HIV, B8/BZLF1 for EBV, and A2/matrix for FLU) and with monoclonal antibodies specific for CD8 and KLRG1. Antibody and tetramer staining was performed as previously described using either heparinized blood, buffy coats, or peripheral blood mononuclear cells isolated from blood by using Ficoll-Hypaque (Amersham Biosciences, Uppsala, Sweden) density centrifugation. Antibodies were obtained from BD PharMingen. Human KLRG1 was detected by Alexa 488-labeled 13A2 monoclonal antibody or by biotinylated or phycoerythrin-labeled 13F12F2 monoclonal antibody (15, 32). Shown are representative dot plots gated on CD8 T cells with percentages of cells present in the different quadrants. (B) Percent KLRG1+ cells of CMV-, HIV-, EBV-, and FLU-specific CD8 T cells identified with the tetramers. Each circle within one particular virus group represents the value from one donor. All donors were analyzed in the chronic (CMV, HIV, and EBV) or postacute (FLU) phase of the infection. KLRG1 expression levels in CMV- and EBV-specific T cells were independent of the HIV status, and FLU-specific T cells were analyzed from HIV-seronegative donors. Peripheral blood mononuclear cells from healthy adult donors (>20 years) were obtained from the Blood Transfusion Center, University Hospital Freiburg, Freiburg, Germany. Further samples were obtained from healthy laboratory workers and from patients chronically infected with HIV, EBV, or CMV, attending clinics in Oxford, United Kingdom. The medical history of each subject was recorded, and blood samples were drawn for serological, virological, and immunological analyses. The study protocol was approved by the local ethics committee.