Abstract
Alzheimer’s disease (AD) causes a characteristic spatiotemporal pattern of neurodegeneration, resulting in the loss of associated faculties such as cognition. The factors which account for this pattern of degeneration are unclear, as AD risk genes are numerous and often broadly expressed. Previously, we generated a model of AD using the nematode Caenorhabditis elegans in which the AD risk variant of apolipoprotein E, APOE4, is pan-neuronally expressed. We showed that HSN class motor neurons degenerate in early adult. Here, we expand on our past work by performing behavioral analyses to deduce the effect of APOE4 on the function of distinct neuronal circuits. We found evidence that APOE4 induces dysfunction of other neurons; this spatiotemporal pattern of degeneration roughly correlates with endogenous levels of PTL-1, the C. elegans homolog of human MAPT also known as tau. Moreover, deletion of ptl-1 suppressed defects in multiple behaviors, suggesting broad protective effects across the nervous system including the HSN neurons. Lastly, we show that PTL-1 in the touch receptor neurons, where PTL-1 is most abundant, is required cell nonautonomously for degeneration of the HSN neurons. Our results suggest that C. elegans may provide a useful in vivo system to study how endogenous Tau acts downstream of APOE4 to cause progressive, patterned neurodegeneration.
Introduction
Neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and frontotemporal lobar dementia (FTLD) present with characteristic spatiotemporal patterns of neuronal cell death (Braak and Braak, 1991; Bahia et al., 2013; Reviewed in Rietdijk et al., 2017). Degeneration in characteristic brain regions corresponds to loss of brain region-associated faculties, such as memory in AD (Rosen et al., 2005). Genetic risk factors have been previously associated with AD. Among them, the ε4 variant of Apolipoprotein E (APOE4) is the most common genetic risk factor for earlier onset and faster progression of sporadic AD and AD-like pathology in individuals with Down syndrome (Larsen et al. 2024; Serrano-Pozo et al., 2021). A recent study proposed that APOE4 homozygosity itself may be a single-gene driver of AD, akin to mutation of the familial AD genes APP, PSEN1, and PSEN2 (Fortea et al., 2024; Ryan and Rossor, 2010). Moreover, APOE4 has been shown to increase the severity of other neurodegenerative diseases such as PD and FTLD (Robinson et al., 2018; Koriath et al., 2019; Davis et al., 2020). How APOE4 worsens neurodegeneration (as in AD, PD, and FTLD) or, more interestingly, may induce it (as suspected in AD) and contribute to patterned degeneration is unclear.
As APOE4 homozygosity alone may cause AD, it is critical to understand how APOE4 contributes to patterned neurodegeneration. One hypothesis is APOE4-induced neurodegeneration follows a spatiotemporal pattern determined by cellular levels of specific vulnerability factors. Multiple lines of evidence suggest that the microtubule associated protein gene, MAPT, which encodes tau, represents one such vulnerability factor. First, pathogenic tau forms intracellular aggregates and their accumulation is closely linked to neuronal dysfunction in AD and other degenerative disorders termed tauopathies (Trojanowski et al., 1993; Lin et al., 2019). Second, Tau-related pathology is highly correlated with neurodegeneration according to Braak Staging, a well-established metric of how AD progresses with age (Braak and Braak, 1991; Köpke et al., 1993; Ghoshal et al., 2002). Third, transgenic expression of pathological tau is sufficient to cause neurodegeneration (Mocanu et al., 2008; Spittaels et al., 1999; www.alzforum.org/research-models). Fourth, depletion of endogenous tau mitigates cellular correlates of AD pathology in rodent models of AD which overexpress human APP (Roberson et al., 2007; Vossel et al., 2010) or human tau with aggregate-inducing mutations (Wegmann et al., 2015; DeVos et al., 2017). Fifth, growing evidence suggests that pathogenic tau protein can spread in a trans-synaptic fashion between brain regions in vivo with age (Liu et al., 2012; de Calignon et al., 2012; Iba et al., 2013; Fu et al., 2016; Meisl et al., 2021; Cornblath et al., 2021). Altogether, these and many other studies provide strong evidence for tau as a candidate vulnerability factor underlying patterned neurodegeneration. Progress has been made to uncover the relationship between APOE4, tau, and neurodegeneration (Shi et al., 2017; Therriault et al., 2020; Seo et al., 2023; Koutsodendris et al., 2023), but whether APOE4 is a causative factor in the pathogenic spread of tau or how APOE4 may contribute to a combination of cell autonomous and non-cell autonomous processes is less clear. Additionally, because rodent models of AD often do not fully recapitulate the neurodegeneration observed in human patients, the cellular molecular bases for this critical pathological phenotype remain have yet to be uncovered.
The nematode Caenorhabditis elegans has been famously leveraged to discover conserved in vivo mechanisms of cell death (Horvitz, 2003). It also represents a potentially useful system to study the cellular molecular bases for patterned neurodegeneration in vivo. To model neurodegeneration, past studies have overexpressed human disease genes and investigated the health and function of specific neurons in vivo (Faber et al., 1999; Baskoylu et al., 2018; van Ham et al., 2008; Vaccaro et al., 2012b). In transgenic C. elegans models, progress has been made to investigate genes involved with AD, notably MAPT and APP (Kraemer et al., 2003; Treusch et al., 2011; Fatouros et al., 2012; Benbow et al., 2020; Sae-Lee et al., 2020). A powerful feature of C. elegans as a model organism is that each one of its 302 neurons is identifiable. However, previous studies did not take full advantage of this to investigate whether specific neurons differ in vulnerability due to tau. Thus, the potential of C. elegans to test spatiotemporal patterns of neurodegeneration remains underutilized.
Here, we found that APOE4 induces a spatiotemporal pattern of neurodegeneration in C. elegans as inferred through the assessment of behaviors which reflect the activity of known neurons. The pattern of neurodegeneration we observed roughly correlates with the neuronal expression levels of ptl-1. Complementing our indirect approach, we observed directly via live microscopy that APOE4 causes progressive, age-related HSN axon dysmorphia coincident with dysfunction of that neuron. Deletion of ptl-1 suppressed both APOE4-induced behavioral defects and HSN axon dysmorphia. Genetic ablation of the mere six neurons most enriched with PTL-1—the touch receptor neurons (TRNs)—similarly suppressed HSN dysfunction; this was phenocopied by targeted depletion of PTL-1 in the TRNs. These results suggest a non-cell autonomous role for PTL-1 in APOE4-induced degeneration. Overall, our findings position the APOE4 worm model as a useful system to study how endogenous tau/PTL-1 participates in the cellular molecular mechanisms underlying patterned neurodegeneration. This may aid in the development of new therapeutic measures against tauopathies, such as AD.
Results
APOE4 induces progressive, widespread behavioral defects in C. elegans
Our previous study using C. elegans showed that pan-neuronal expression of APOE4 induced degeneration of a subset of neurons (the HSN pair) in early adult, while others (such as VA and VB class neurons) appeared to remain healthy (Sae-Lee et al., 2020). To expand on this study, we investigated whether APOE4 induces a wider pattern of neurodegeneration. We assayed a variety of behaviors to assess the function of different subsets of neurons. We reasoned that we could deduce the health and function of different neuronal subsets and circuits by measuring the performance of these behaviors.
We selected seven different behaviors that rely primarily, but not exclusively, on mostly distinct subsets of identified neuron classes (Fig. S1). Five behaviors we tested—egg laying, pharyngeal pumping, gentle and harsh touch response, and locomotor gait transition—have specific neurons which are critical for behavioral execution. These include: the HSN class neurons for egg laying behavior (Schafer, 2005); the MC and M3 neurons for pumping of the pharyngeal feeding organ (Avery and You, 2012); the six touch receptor neurons (TRNs) for gentle touch response (Chalfie and Sulston, 1981; Chalfie et al., 1985); the FLP and PVD neurons, with lesser contribution from redundant mechanoreceptive neurons and interneurons, for harsh touch response (Li et al., 2011); and lastly, the ADE and PDE dopaminergic neurons for the transition between swim and crawl locomotor gaits (Vidal-Gadea et al., 2011). We also assessed two readouts of locomotor behavior that rely on many more neurons with partial redundancy. Short-term locomotion relies on over 100 motor neurons and about one dozen pre-motor interneurons; long-term locomotion relies on those neurons and additional sensory neurons and interneurons to mediate switches between roaming and dwelling states (Flavell, et al., 2020).
We evaluated behavioral performance at adult days 1 through 5 (D1–5) (Fig. 1A, left). This age range encompasses the peak reproductive period when C. elegans is active across a range of behaviors and lays eggs (Scharf et al., 2021). We refer to “age of onset” of behavioral decline as the first day of the adult stage that APOE4 worms displayed significantly impaired behavior compared to age-matched controls.
Figure 1. ptl-1 is required for widespread, progressive neuronal dysfunction induced by APOE4.
(A left) Timeline from egg lay to the adult age of onset at which defective or abnormal performance in APOE4 versus control animals are first detected.
(A right) Endogenous ptl-1 locus with all known isoforms and the portion deleted in the ok621 allele as indicated below.
(B-H) Results of behavioral assays, used to proxy neuronal dysfunction, which are arranged by the adult age of onset for APOE4-induced defective or abnormal performance.
(B-E) D1 adult onset. (B) Subtle defect in gentle touch sensitivity which is fully suppressed by ptl-1 deletion. N=47 for control, N=30 for Δptl-1, N=33 for APOE4, and N=35 for APOE4;Δptl-1. (C) Subtle defect in pharyngeal pumping which is fully suppressed by ptl-1 deletion. N=94 for control, N=37 for Δptl-1, N=61 for APOE4, and N=91 for APOE4;Δptl-1. (D) Severe defect in swim-to-crawl gait transition which is partially suppressed by ptl-1 deletion. N=164 control, N=136 for Δptl-1, N=162 for APOE4, N=165 for APOE4;Δptl-1. (E) Subtle defect in short-term locomotion which is not suppressed by ptl-1 deletion. N=47 for control, N=38 for Δptl-1, N=44 for APOE4, and N=44 for APOE4;Δptl-1).
(F) D2 adult onset. Subtle defect in long-term locomotion which is not suppressed by ptl-1 deletion. N=31 control, N=32 for Δptl-1, N=28 for APOE4, and N=29 for APOE4;Δptl-1.
(G,H) D3 adult onset. (G) Severe egg laying defect which is partially suppressed by ptl-1 deletion. N=196 for control, N=134 for Δptl-1, N=214 for APOE4, N=243 for APOE4;Δptl-1. (H) Subtle, abnormal increase in harsh touch sensitivity. N=25 for control, N=17 for Δptl-1, N=26 for APOE4, and N=31 for APOE4;Δptl-1.
All data are represented as mean ± SEM. N is the total sample size representing all animals across replicates. Statistical comparisons were made using χ2 tests of independence for D,G and Student’s t-test for B,C,E,F,G. *: p<0.05; **: p<0.01; ***: p<0.001.
Over the course of D1–5 of adult, we found that APOE4 worms displayed differences in all seven behaviors compared to control (Fig. 1B-H). In as early as D1 adults, gentle touch sensitivity, pharyngeal pumping, and short-term locomotion performances decreased by 11%, 13%, and 24%, respectively; gait transition decreased by a marked 59% (Fig. 1B-E). In D2 adults, long-term locomotion showed a 12% decline. We also replicated our previous finding of an egg-laying defect which leads to larvae hatching internally, a salient phenotype called “bagging” (Sae-Lee et al., 2020; Fig. 1G). APOE4 worms showed a salient 60% incidence of bagging, peaking at D3. Whereas other behavioral performances declined, harsh touch sensitivity oddly increased in APOE4 worms relative to control beginning at D3 (Figs. 1H, S2). Altogether, these results indicate that APOE4 induces age-related abnormalities in multiple behaviors which primarily rely on distinct subsets of neurons. From this observation, we conclude that the APOE4 worm exhibits a wider spatiotemporal pattern of neurodegeneration, beyond the HSN neuron pair, across early worm adult age.
APOE4-induced progressive behavioral defects require the worm ortholog of tau
Above, we reported that gentle touch response is among the first behaviors to decline with APOE4 on D1 adult. This behavior is mediated by the six touch receptor neurons (TRNs) which feature a prominent array of microtubules that were exploited to discover the first microtubule gene (Chalfie and Thomson, 1979; Chalfie and Thomson, 1982). Studies have shown that tau binds to and stabilizes neuronal microtubules in mammals (Butner and Kirschner, 1991; Santarella et al., 2004). As one may expect from microtubule-enriched neurons, the worm homolog of tau, PTL-1, is highly enriched in the TRNs compared to other neurons, notably the PLM and ALM classes, according to recent single-cell transcriptomic analyses (Taylor et al., 2021; Roux et al., 2023; Fig. S3). We also noticed a rough correlation between endogenous levels of PTL-1 in specific neurons across the nervous system and the age of onset for APOE4-induced behavioral decline. This is represented in Fig. S1, where gray shows what fraction of ptl-1 is expressed in critical neurons that subserve each behavior. Given that tau represents a pathological hallmark associated with neurodegeneration in AD, we speculated that the high endogenous levels of PTL-1 in the TRNs may explain the early onset for the APOE4-induced gentle touch defect. Following this idea, we also hypothesized that deletion of ptl-1 may confer neuroprotection against APOE4. Into an APOE4 background we crossed a ptl-1 mutant bearing the ok621 deletion allele (Δptl-1). This allele represents a strong hypomorph, if not a null allele, as it lacks four exons that encode conserved microtubule-binding domains important for PTL-1 function (Goedert et al., 1996; Gordon et al., 2008; Hashi et al., 2015; Figs. 1A right, S4).
We found that deletion of ptl-1 significantly suppressed APOE4-induced defects in four out of seven behaviors. We observed complete suppression of defects in gentle touch and pharyngeal pumping (Fig. 1B,C), with partial suppression for defects in gait transition and egg laying (Fig. 1D,G). We did not observe suppression of APOE4-induced abnormalities in short- and long-term locomotion nor for harsh touch sensitivity (Fig. 1E,F,H).
The behavioral performance of the ptl-1 null mutant was also indistinguishable from control across six out of seven behaviors. Although the ptl-1 null mutant displayed a moderate defect in swim-to-crawl transition compared to control, the ptl-1 null mutant nonetheless partially suppressed the defect induced by APOE4 as opposed to additively worsening the defect (Fig. 1D). Also, although ptl-1 deletion led to a non-significant trend toward lower bagging compared to control animals, it caused a significant suppression of the APOE4-induced bagging (Fig. 1G).
Altogether, we conclude that PTL-1 broadly contributes to the neuronal dysfunction given significant and multiple behavior changes observed in the presence of APOE4.
APOE4-induced degeneration of HSN axon requires worm ortholog of tau
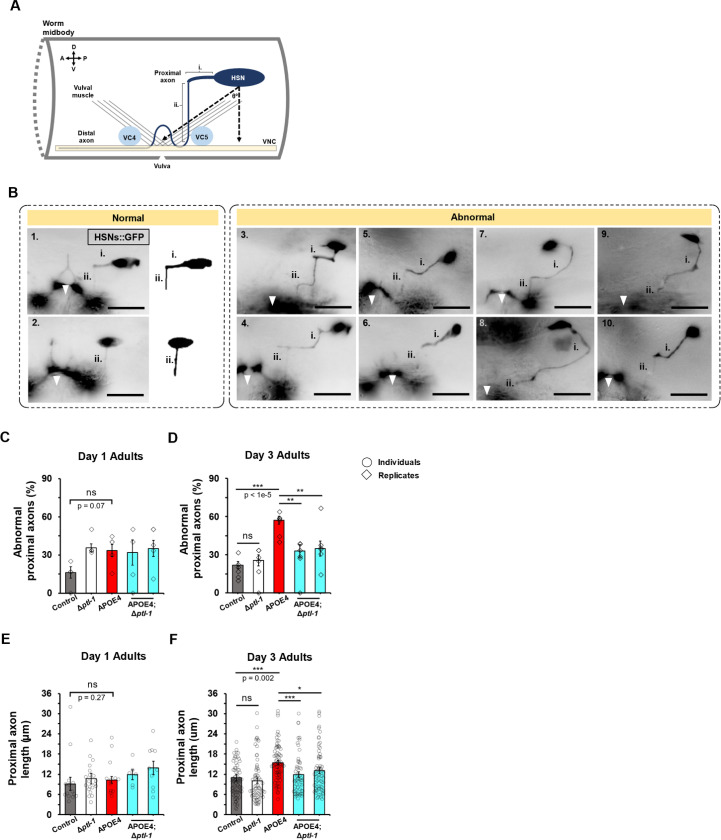
Previously, we showed that APOE4-induced bagging coincided with degeneration of the bilaterally symmetric pair of HSN egg-laying motor neurons (Sae-Lee et al., 2020). Because deletion of ptl-1 suppressed APOE4-induced bagging, we hypothesized that ptl-1 deletion would also suppress HSN neurodegeneration. To test this, we inspected the health of reporter-labeled HSN neurons in live worms with epifluorescent microscopy. Dorsal to the vulva, which is the egg-laying orifice, the HSNs are situated in the midbody of the worm (depicted in Fig. 2A). To quantify degeneration, we initially calculated various metrics of HSN soma geometry and reporter intensity across strains (Fig. S5A), focusing on D3 adult animals which is the age of onset for increased bagging (~70% incidence in APOE4 worms; Fig. 1G).
Figure 2. ptl-1 is required for APOE4-induced HSN axon degeneration.
(A) Cartoon of the worm midbody, centered about the vulva area, depicting: the HSN proximal axon, the VC4,5 neurons, vulval musculature, and ventral nerve cord (VNC).
(B) Representative live images of normal (1,2) and abnormal (3–10) HSN proximal axons visualized with GFP. Proximal axon segments are marked by i. and ii (with exception to image 2, where segment identity is less certain). The vulva area is also marked with a white arrowhead for anatomical reference. All scale bars are 20 μm.
(C,D) Percentage of animals with abnormal HSN proximal axons in D1 and D3 adults. The baseline incidence of abnormal axons in control was similar between D1 and D3.
(C) D1 adults. All other groups show non-significant, but slightly increased incidence of abnormal axons compared to control. N=37 for control, N=59 Δptl-1, N=51 for APOE4, N=22 for APOE4;Δptl-1 (left), and N=37 for APOE4;Δptl-1 (right).
(D) The incidence of abnormal axons appears to correlate with defective egg laying behavior (See Fig. 1G) in D3 adults. N=101 for control, N=78 for Δptl-1, N=91 for APOE4, N=79 for APOE4;Δptl-1 (left), and N=86 for APOE4;Δptl-1 (right).
(E,F) Quantification of HSN proximal axon lengths in D1 and D3 adults. The baseline axon length in control was similar between D1 and D3.
(E) D1 adults. Axon length was similar across all groups. N=15 for control, N=13 Δptl-1, N=20 for APOE4, N=6 for APOE4;Δptl-1 (left), and N=10 for APOE4;Δptl-1 (right).
(D) D3 adults. Axon length appears to correlate with the incidence of abnormal axons shown in (C) and defective egg laying behavior (See Fig. 1G). N=79 for control, N=66 for Δptl-1, N=72 for APOE4, N=54 for APOE4;Δptl-1 (left), and N=66 for APOE4;Δptl-1 (right).
All data are represented as mean ± SEM. N is the total sample size representing all animals across replicates. Statistical comparisons were made using χ2 tests of independence for C and D and Student’s t-test for (E) and (F). *: p<0.05; **: p<0.01; ***: p<0.001.
Consistent with our previous study (Sae-Lee et al., 2020), we found that the HSN soma appeared on average much dimmer in APOE4 than control worms, indicative of degeneration (Fig. S5D). However, deletion of ptl-1 alone also appeared to have caused dimming along with abnormally large appearance of the HSN soma (Fig. S5B,D). We found no straightforward relationship between other soma morphometrics and HSN dysfunction as reflected by bagging incidence across control, ptl-1, and APOE4 strains (compare Fig. 1G to Fig. S5 measures). Thus, we conclude that HSN soma geometry and brightness are inconsistent predictors of HSN function in these strains. possibly due to unforeseen aspects of the fluorescent reporter (see Methods).
Seeking a more reliable cellular correlate of HSN function, we next investigated HSN axon morphology. Previous work using laser ablation and mutants showed the proximal axon portion (located between soma and vulval muscles)—but not distal portion which continues anteriorly—is required for egg laying behavior (Garriga et al., 1993; Fig. 2A). Therefore, we hypothesized that morphology of the proximal axon may serve as a useful correlate of HSN neuron function.
We visualized the HSN proximal axon with the same fluorescent reporter as above for the soma. The ~11 μm-long HSN proximal axon typically comprises two segments joined at a right-angle (Fig. 2B image 1). The initial segment, which is qualitatively thicker, projects anteriorly from the soma (segment i.) before turning ventrally at a right-angle into a second, thinner segment (segment ii.) that joins the ventral nerve cord (VNC) and branches dorsally to innervate egg-laying musculature. The unbranched, distal axon continues anteriorly within the VNC to the head (Fig. 2A; Garriga et al., 1993). In our control reporter strain, we found that ~58% of worms had a proximal axon with this typical two-segment, right-angle morphology (N=93; Fig. 2B image 1), while the remainder had a single ventrally-projecting proximal axon from the soma (Fig. 2B image 2). The latter single-segmented axons were thin and resembled segment ii. of two-segment HSN proximal axons. We speculate that the thicker, anteriorly directed segment i. did not develop in these worms because the HSN soma had already migrated to a position directly dorsal from the vulval muscles. Although the HSN proximal axon has been previously depicted with two segments connected at a right angle (Asakura et al., 2007; Tsutsui et al., 2021), both configurations have been described in the literature and are functional (Garriga et al., 1993). Thus, both types of proximal axons were scored as normal.
To check for axon abnormalities potentially caused by APOE4, we next qualitatively scored the morphology of HSN proximal axons and quantified their lengths in D3 adult animals. We found that the incidence of abnormal proximal axons was more than double in APOE4 compared to control worms. Proximal axons often took eccentric, elongated paths in APOE4 worms (compare Fig. 2B normal images 1,2 to abnormal images 3–10). This was not easily explained by abnormal positioning of the HSN soma, as the somas were similarly located in both APOE4 and control worms; soma positions were quantified as the HSN soma to VNC distance and the HSN soma to vulva area distance (Fig. S6C left) or the angle formed between these two vectors (Fig. S6C right; Asakura et al., 2007).
We observed at least three distinct qualitatively abnormal morphologies of the proximal axons relative to control. First, segment i. (Fig. 2B, image 3) or segment ii. (Fig. 2B, image 4) had an extra ~90-degree turn. Second, segment i. connected to segment ii. with a typical right-angle turn, but displayed an atypical ~45-degree portion, with an apparently wider segment near the soma (Fig. 2B images 5,6). Third, segments i. and ii. together took on a hook-like shape oriented anteriorly (Fig. 2B images 7,8). We also noted additional cases (<5% frequency) where these morphological abnormalities appeared to co-occur (e.g., segment ii. extra right-angle turn and segment i. 45-degree portion, Fig. 2B, image 10). Overall, we conclude that APOE4 appears to induce a variety of proximal axon abnormalities of the HSN neurons.
To help distinguish whether the HSN proximal axon abnormality represented a developmental defect, age-related degeneration, or both, we inspected younger adult animals to determine when the defect started. By at least D1, there was a non-significant trend toward increased abnormal axons in APOE4 worms versus control (Fig. 2C). However, in D3 adults, the percentage of abnormal axons showed a significant three-fold increase in APOE4 worms versus control (Fig. 2D). From these observations, it appears that the proximal axon defect begins by at least D1 in some APOE4 worms but progresses in an age-related fashion, concurrent with the age of onset for increased bagging (Fig. 1G). We thus conclude that the increased incidence in axon abnormality primarily reflects degeneration.
We next tested whether deletion of ptl-1 might suppress APOE4-induced abnormal HSN proximal axon morphology. To partly control for genetic background, we inspected two independent isolates of APOE4;Δptl-1 worms that were crossed with the HSN reporter strain. For both isolates, we found that the ptl-1 deletion suppressed the APOE4-induced abnormal axon morphology (Fig. 2D). Moreover, although the incidence of axon abnormality was slightly higher in the ptl-1 deletion mutant at D1, the axon defect was nonetheless significantly suppressed by ptl-1 deletion in D3 adults (Fig. 2C,D).
The above results support the idea that normal proximal HSN axon morphology is a reliably predictive correlate of efficient egg-laying behavior and thus HSN health and function. Specifically, the similar incidence of axon abnormality and bagging in APOE4 worms led us to conclude that abnormal proximal axons reflect a defect in HSN function (compare control to APOE4 in Figs. 1G, 2D). Finally, we found that deletion of ptl-1 is protective against age-related APOE4-induced defects in behavioral performance as well as HSN proximal axon morphology.
Ablating tau-rich touch receptor neurons protects HSN from APOE4-induced dysfunction
The spatiotemporal pattern of degeneration in AD is thought to be partly explained by pathological forms of tau spreading between connected neurons and across the brain (intra- and extra-synaptically; Liu et. al, 2012; de Calignon et al., 2012). As shown above, the systemic deletion of ptl-1 protected against age-related APOE4-incuded behavioral defects and abnormal HSN proximal axon morphology. We next sought to test whether PTL-1 might contribute non-cell autonomously to degeneration, explaining in part the spatiotemporal pattern of degeneration in our APOE4 worm model. We hypothesized that targeting the most PTL-1-abundant neurons which— tend to become dysfunctional early—may suppress the degeneration of other neurons that express PTL-1 at a lower level and become dysfunctional later. The TRNs represent the neuronal class most abundant in PTL-1 in C. elegans. Over 30% of neuronal ptl-1 transcripts are expressed in the mere six TRNs (Fig. S1,S3; Taylor et al., 2021; Roux et al., 2023). Coincidentally, the TRNs also become dysfunctional in D1 adults (Fig. 1B). In contrast, the HSN neurons express ~80% less ptl-1 than the TRNs and become dysfunctional later starting in D3 adults (Figs. 1G, S3; Taylor et al., 2021). Some TRNs are also nearby the HSN neurons, such as the PLMs which synapse onto the HSN neurons (White et al., 1986). Thus, the TRNs and HSN neurons in worm offer a convenient platform to test whether PTL-1 contributes non-cell autonomously to degeneration.
To assess the TRNs and PTL-1 protein in vivo during our targeted manipulations, we took advantage of a control strain in which endogenous PTL-1 protein translationally tagged with mNeonGreen (PTL-1::mNG; Krieg, et al., 2017). Consistent with transcriptional studies, TRNs were readily visible in living larval and adult stages by their abundant mNG-tagged PTL-1 (Representative PLM neuron in Figs. 3A2,4B2). We also tested whether the mNG tag on PTL-1 impedes its apparent neurotoxic properties downstream of APOE4. If this were the case, we might expect less HSN dysfunction in APOE4;PTL-1::mNG worms. Instead, we found bagging incidence in APOE4;PTL-1::mNG worms (60%) was similar to that of the APOE4-alone strains generated in the current (~70%) and our previous (50–60%) studies (note: all APOE4 strains depicted in Fig. 3A1 express mNG::PTL-1, versus those in Fig. 1G which do not; Sae-Lee et al., 2020). We conclude that the endogenous mNG tag does not interfere with the presumed toxicity of PTL-1 in an APOE4 background.
Figure 3. Targeted genetic ablation, but not abolished function, of the TRNs protects HSN against APOE4-induced dysfunction.
(A1) Egg-laying behavior across groups. Genetic ablation of the TRNs partially suppressed egg-laying defects in APOE4 animals as with the ptl-1 deletion (See Fig. 1G). N=104 for control, N=171 for TRNs::EGL-1, N=525 for APOE4, and N=190 for APOE4;TRNs::EGL-1.
(A2 top) Cartoon depicting the head and tail areas wherein the nerve ring and PLM neuron, respectively, are located.
(A2 bottom) Representative live images of nerve ring (arrowhead) and PLM neuron (within dashed circle if present; marked with asterisk if absent) visualized with PTL-1::mNG in control and TRNs::EGL-1 animals. Dashed borders indicate the worm body outline. All scale bars are 20 μm.
(B) Egg-laying behavior across groups. In contrast to genetic ablation, abolishing TRN function did not suppress egg laying defects. N=104 for control, N=123 for mec-4(lf), N=525 for APOE4, and N=101 for APOE4;mec-4(lf) (left), and N=125 for APOE4;mec-4(lf) (right).
All data are represented as mean ± SEM. N is the total sample size representing all animals across replicates. All statistical comparisons were made using χ2 tests of independence. control and APOE4 data are re-plotted in (A1) and (B). *: p<0.05; **: p<0.01; ***: p<0.001.
Figure 4. Targeted depletion of PTL-1 in the TRNs protects HSN against APOE4-induced dysfunction.
(A1) Egg-laying behavior across groups. N=104 for control, N=180 for TRNs::ptl-1 KD, N=525 for APOE4, N=171 for APOE4;TRNs:: ptl-1 KD (Left), and N=108 for APOE4;TRNs:: ptl-1 KD (Right).
(A2) Quantification of ptl-1 knockdown in the targeted TRNs and non-targeted VCs4,5 neurons between control and TRNs::ptl-1 KD animals using PTL-1::mNG fluorescence intensity in the soma.
(B1) Egg-laying behavior across groups. N=104 for control, N=348 for TRNs::ptl-1 KO, N=525 for APOE4, N=423 for APOE4;TRNs:: ptl-1 KO (Left), and N=519 for APOE4;TRNs:: ptl-1 KO (Right).
(B2) Representative live images of nerve ring (arrowhead) and PLM neuron (within dashed circle if present; area marked with asterisk if absent) visualized with PTL-1::mNG in control and TRNs::ptl-1 KO animals. Dashed borders indicate the worm body outline. All scale bars are 20 μm.
All data are represented as mean ± SEM. N is the total sample size representing all animals across replicates. Statistical comparisons were made using χ2 tests of independence for A1,B1 and Student’s t-test for A2. Control and APOE4 data are re-plotted in (A1) and (B1). *: p<0.05; **: p<0.01; ***: p<0.001.
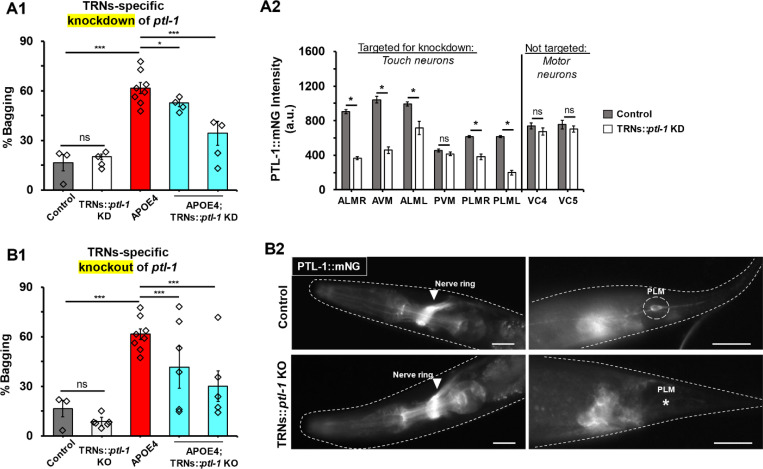
For our first targeted manipulation, we tested whether ablation of the TRNs suppresses APOE4-induced HSN dysfunction. To do this, we leveraged the apoptotic trigger EGL-1, the sole worm homolog of the human BH3-only domain proteins BAM and BID. Targeted expression of egl-1 has been previously used to kill specified cells (Chang et al., 2006). To kill the TRNs, we generated a strain whose egl-1 expression is driven by the promoter of mec-17, a gene expressed exclusively in the TRNs (TRNs::EGL-1; Wu et al., 2025). The TRNs::EGL-1 strain was then crossed into the mNG-tagged ptl-1 genetic background. To confirm the efficacy of TRN genetic ablation, we took advantage of the relative abundance of mNG-tagged PTL-1 in the TRNs (Fig. S3), which could be directly observed using epifluorescence microscopy (Fig. 3A2 control). Anatomically, this was easier to test with the PLM class of TRNs due to how its soma and process are confined in the narrow tail region, in contrast with the other TRN classes with soma situated in the thicker midbody (Fig. 3A2 control; WormAtlas). We found that 93% of TRNs::EGL-1 animals had no detectable PTL-1::mNG where PLM soma and processes were expected compared to control (N=46; Fig. 3A2), suggesting a successful cell ablation in most cases.
We found that ablating the TRNs partially suppressed APOE4-induced HSN dysfunction as indicated by a ~30% reduction in bagging incidence (yellow bar, Fig. 3A1). Ablation of the TRNs alone in the control strain had no effect on bagging (white bar, Fig. 3A1). Impressively, ablating the TRNs conferred a similar magnitude of protection as afforded by systemic deletion of ptl-1 (compare blue bar in Fig. 1G to yellow bar in Fig. 3A1). Thus, we deduce that TRNs activity and/or their cellular contents may contribute non-cell autonomously to APOE4-induced HSN degeneration.
Because TRN activity is obviously lost after genetic ablation, we asked whether simply blocking TRNs function alone as opposed to whole cell ablation may protect the HSNs against APOE4. We tested this by using a deletion allele of mec-4, a gene which encodes mechanoreceptive DEG/ENaC channel expressed predominantly in the TRNs (Chalfie and Sulston, 1981). We took advantage of the u253 loss-of-function allele of mec-4, referred hereon as mec-4(lf), which abolishes transduction of mechanosensory currents and gentle touch response yet leaves the TRNs otherwise physiologically and morphologically normal (O’Hagan, et al., 2005). We found that mec-4(lf) failed to suppress APOE4-induced HSN dysfunction (Fig. 3B). We conclude that functional impairment of the TRNs is insufficient to protect HSNs against APOE4.
Targeted reduction of tau in touch receptor neurons protects HSN against APOE4
Because the worm ortholog of tau, PTL-1, is especially abundant in the TRNs (green bars in Fig. S3), we hypothesized that targeted depletion of PTL-1 contained in the TRNs would protect against APOE4-induced HSN dysfunction, phenocopying TRNs ablation. To test this, we knocked down ptl-1 expression in the TRNs using cell-selective RNAi as previously described (TRNs::ptl-1 KD; Esposito et al., 2007). To determine the efficacy and selectivity of the knockdown, we quantified PTL-1::mNG fluorescence intensity in TRN soma versus example non-targeted neurons. Conveniently, the soma of the non-targeted VC4 and VC5 class motor neurons (depicted in Fig. 2A) are also easily detectable by PTL-1::mNG fluorescence. Compared to control, TRNs::ptl-1 KD worms had an average 40% decrease in PTL-1::mNG fluorescence in five out of the six TRNs (Fig. 4A2). In contrast, PTL-1::mNG fluorescence intensity was unchanged in the non-targeted VC4 and VC5 neurons (Fig. 4A2). These observations suggested an effective and selective knockdown of ptl-1 in the TRNs.
Using this strategy, we found that knockdown of ptl-1 in the TRNs partially suppressed APOE4-induced HSN dysfunction. This was determined after scoring bagging in two independent isolates of APOE4 worms crossed with the TRNs::ptl-1 KD strain to partly control for genetic background (blue bars, Fig. 4A1). In contrast, knockdown of ptl-1 in TRNs alone in the control strain had no effect on bagging (white bar, Fig. 4A1). While significant, the suppressive effect was noticeably weaker compared to the systemic ptl-1 deletion (compare with APOE4;Δptl-1 strain, blue bar in Fig. 1G). We speculated that the weaker suppression may be explained by RNAi reducing only a portion of ptl-1 transcripts in the TRNs (Fig. 4A2). This prompted us to test whether complete loss of ptl-1 in the TRNs more strongly suppresses HSN dysfunction than in APOE4;TRNs::ptl-1 KD worms.
To achieve a targeted ptl-1 knockout in the TRNs, we used an intersectional Cre-LoxP approach. LoxP sites were first introduced via CRISPR to flank the endogenous ptl-1::mNG locus (LoxP::ptl-1::LoxP strain). LoxP::ptl-1::LoxP worms were then crossed with a strain which expresses Cre recombinase under the same TRNs-specific promoter as our TRNs::EGL-1 strain (Harterink et al., 2018; Sanfeliu-Cerdán et al., 2023; Wu et al., 2025). This should result in a selective knockout of ptl-1 in the TRNs (TRNs::ptl-1 KO). The efficacy of the ptl-1 knockout was confirmed by evaluating the presence of PTL-1 in the PLM neurons via PTL-1::mNG fluorescence signal (Fig. 4B2). PTL-1::mNG was absent in 100% of PLM neurons in TRNs::ptl-1 KO animals (N=45). However, we were unable to detect significant reduction in mNG-tagged PTL-1 protein as evaluated by Western blot probing for the FLAG epitope engineered into the tag (Fig. S7; Krieg et al., 2017).
As with the knockdown, we found that knockout of ptl-1 in the TRNs partially suppressed APOE4-induced HSN dysfunction. This was determined after scoring bagging in two independent isolates of APOE4 worms crossed with the TRNs::ptl-1 KO strain to partially control for genetic background (blue bars in Fig. 4B1). In contrast to the mild suppression after ptl-1 knockdown (blue bars in Fig. 4A1), the average extent of suppression by knockout in the TRNs was more equivalent to that of the systemic deletion of ptl-1 (Fig. 1G).
Altogether, these findings suggest that PTL-1 is required in the TRNs to contribute non-cell autonomously to APOE4-induced degeneration of the HSN neurons.
Discussion
APOE4-induced pattern of neurodegeneration in C. elegans
In this study, we build on our prior work by characterizing APOE4-induced, age-related abnormalities across seven behaviors in a C. elegans model of Alzheimer’s disease (AD). The pattern of decline provides evidence for spatiotemporal APOE4-induced neurodegeneration. Finally, we demonstrate that endogenous tau contributes to APOE4-induced degeneration and multiple behavioral defects.
C. elegans has served as a leading model organism to study forms of cell death (Horvitz 2003); however, its potential to study patterned neurodegeneration in response to human disease genes remains to be explored. This prompted us to ask whether APOE4 worms display a pattern of neurodegeneration beyond the HSN neurons as we previously reported (Sae-Lee et al., 2020). By day 4 (D4) of adulthood, APOE4 worms exhibit defects of varying severity in all seven tested behaviors. Because each behavior primarily relies on mostly distinct subsets of neurons (Fig. S1), this suggests a progressive, neuron-specific pattern analogous to the characteristic progression observed in human AD (Braak and Braak, 1991; Scahill et al., 2002; Fiford et al., 2018). The sensitivity of certain behaviors to decline merits follow up studies to investigate putative higher-vulnerability neurons (e.g., the ADE and PDE neurons required for swim-crawl gait transition; Fig. 1D; Vidal-Gadea et al., 2011). Certain behaviors were not impacted by pan-neuronal APOE4 expression; this may reflect how certain neurons are resilient to PTL-1-mediated degeneration. This idea is mirrored in human AD where brain regions such as the somatosensory cortex and brainstem are similarly spared from degeneration, even in advanced stages of AD (Rondina et al., 2018). Alternatively, apparent resistance to APOE4 may reflect neuronal circuit redundancy. In this vein, the subtle defects we observed in locomotion could reflect the suspected functional redundancy of C. elegans motor neurons (Fig. 1E,F; Izquierdo and Beer, 2018). Interestingly, harsh-touch sensitivity subtly increased in APOE4 worms, hinting at neuronal hyperexcitability (Fig. 1H). In mouse, APOE4-driven hyperexcitability may result from degeneration of inhibitory neurons (Nuriel et al., 2017a). This aligns with fMRI studies of AD patients showing abnormally high activity in certain brain regions (Bookheimer et al., 2000; Tuovinen et al., 2020). Future studies could explore how APOE4 alters excitability of harsh touch neurons (e.g., the PVDs) and how this may correlate with APOE4-induced progressive dysmorphia of the elaborate PVD dendrite arborization (Jiang et al., 2025).
Axon dysmorphia, or axonopathy, is a hallmark of AD characterized by neurite deterioration and general white matter abnormalities (Lee et al., 2016). Similar axon defects, sometimes without cell body loss, have been observed in human AD patients and mouse neurodegenerative disease models (Leroy et al., 2007; Yoshiyama et al., 2007; Crespo-Biel et al., 2014). Here, we identified APOE4-induced dysmorphia of the HSN proximal axon (Fig. 2). Our findings suggest this reflects a degenerative phenotype rather than a developmental defect, as egg laying behavior as well as HSN soma position and axon shape are all initially normal in D1 versus D3 adult APOE4 worms (Figs. 2B,C,D, S6). Worm neurons have been shown to contain axonal domains with distinct structures and functions (Mizumoto and Shen, 2013; Eichel et al., 2022). Future studies should consider what molecular factors might render the proximal HSN axon vulnerable to dysmorphia. Overall, the tight correlation between a salient behavioral phenotype (bagging; Fig. 1G) and axon dysmorphia of an identified neuron (HSN; Fig. 2) provides a powerful system to study subcellular mechanisms of APOE4-induced axonopathy.
C. elegans has become increasingly recognized as a valuable in vivo model for studying tau pathology (Reviewed in Natale et al., 2020). Along with extracellular amyloid-β plaques, intracellular tau aggregation is a key pathological hallmark of AD (Kowall and Kosik, 1987; Kosik et al., 1988; Grunke-Iqbal et al., 1986; Lee and Trojanowski, 1992). In AD and other tauopathies, tau undergoes conformational changes that promote aggregation into tangles, driving neurodegeneration (Friedhoff et al., 1998a,b; Jeganathan et al., 2006; Jeganathan et al., 2008; Oakley et al., 2020). Unlike mammals, which have three broad tau-related gene families, the worm has but a single endogenous homolog: ptl-1 (Sündermann et al., 2016; Goedert et al., 1996; Gordon et al., 2008). We found that deletion of ptl-1 suppresses APOE4-induced defects in four out of seven tested behaviors—including HSN-associated bagging—and HSN axon morphological defects induced by APOE4. This fits with the idea that tau acts as an effector of pathogenic events in AD initiated by APOE4. Indeed, past immunohistochemical evidence suggested that APOE and tau co-localize – and possibly physically interact – in postmortem brain tissue from individuals with Alzheimer’s disease (Benzing and Mufson, 1995; Brecht et al., 2004; Zhou et al., 2006; Rohn et al., 2012). Further studies showed that APOE4 expression induces tau aggregates in cultured mouse neurons and worsens tau pathology in an AD mouse model (Huang et al., 2001; Seo et al., 2023; Koutsodendris et al., 2023 Shi et al., 2017). It will be interesting to see whether endogenous worm PTL-1 has similar pathological capacity detectable at the biochemical level.
We found that deletion of endogenous ptl-1 suppresses APOE4-induced degeneration. This parallels a previous report in a mouse AD model in which APOE4-induced dysfunction of specific interneurons was suppressed by knocking out endogenous murine tau (Andrews-Zwilling et al., 2010). Interestingly, another study found that depletion of endogenous tau protected against neurodegeneration in an amyloid-β mouse model (Roberson et al., 2007). Because C. elegans lacks an APP ortholog whose protein product is capable of generating amyloid-β fragments (Daigle and Li, 1993), the benefits of PTL-1 reduction likely operate independently of amyloid-β. Interestingly, a recent study found that ptl-1 deletion partly mitigated age-dependent accumulation of pathogenic human Tau upon its overexpression in worm neurons (Nunez et al., 2022). Thus, the APOE4 worm model of AD represents an Aβ-agnostic model to investigate how endogenous tau contributes to patterned degeneration downstream of APOE4.
PTL-1 may contribute cell non-autonomously to degeneration in our APOE4 worm model. The touch receptor neurons (TRNs) contain the majority of PTL-1 in the worm nervous system (green bars in Figs. 3A2, 4A2; Taylor et al., 2021; Roux et al., 2023). We found that depleting PTL-1 in the TRNs via genetic ablation, RNAi-mediated knockdown, and Cre-LoxP-mediated knockout all partially suppressed APOE4-induced dysfunction of HSN neurons. Each targeted manipulation roughly matched the magnitude of suppression achieved by systemic ptl-1 deletion. This raises the intriguing possibility that, although ptl-1 remains widely expressed, PTL-1 from the mere six TRNs is required cell non-autonomously for HSN dysfunction.
How might PTL-1 in the TNRs lead to APOE4-induced degeneration of the HSNs? The popular hypothesis that pathological tau spreads across the brain offers a partial link between tau pathology and spatiotemporal neurodegeneration (Braak and Braak, 1991; Reviewed in Braak and Del Tredici, 2011; Frost et al., 2010). Tauopathy mouse models show that pathological tau can propagate across synapses, starting from “seed” regions rich in Tau (and high in inter-neuronal connectivity) and spreading to distant brain regions (Clavaguera et al., 2009; Kfoury et al., 2012; Liu et al., 2012; de Calignon et al., 2012; Iba et al., 2013). Similarly, we hypothesize that pathological PTL-1 may spread from neuron to neuron over time in worm. Although the six TRN soma are relatively distant from the two HSN soma (1/2–1/3 body length or ~30–50 mm), PTL-1 is also enriched in TRN processes which pass nearby the HSNs (Fig. 3A2). Two TRNs (the PLM pair) also form chemical synapses with the HSNs (White et al., 1986). Future work could leverage synaptic transmission mutants to test whether this represents a site of PTL-1 spread (Miller et al., 1996; McColluch et al., 2017). If the hypothetical PTL-1 spread is synaptic, genetically perturbing TRN neurotransmission may phenocopy our targeted manipulations described above, suppressing HSN dysfunction. Moreover, we found that fluorescently tagged PTL-1 did not interfere with PTL-1-dependent degeneration of the HSNs (compare APOE4 strains with mNG tagged PTL-1 in Figs. 3,4 to non-tagged PTL-1 in Fig. 1G). This raises the possibility of directly visualizing PTL-1 spread in vivo. With its simpler and fully mapped nervous system, C. elegans offers a simple model to investigate how endogenous tau propagates in vivo and contributes to APOE4-induced patterned neurodegeneration.
Materials and Methods
Strain Maintenance
Worms were maintained at room temperature (20 °C) on NGM plates seeded with OP50 bacteria as described (Brenner et al. 1974). Strains with extrachromosomal transgenes were propagated using selectable fluorescent reporters. Strains are listed in order of appearance in figures:
Figures 1 and S2
JPS1451 vxEx1211[Pmyo-3::mCherry::UNC54UTR]
RB809 ptl-1(ok621) III
JPS1453 vxEx1213[Prab-3::ND18ApoE4::UNC54UTR + Pmyo-3::mCherry::UNC54UTR]
JPS1455 vxEx1215[Prab-3::ND18ApoE4::UNC54UTR + Pmyo-3::mCherry::UNC54UTR]; ptl-1(ok621) III
Figures 2, S5, and S6
JPS1666 vxIs591[vxIs591[ptph-1::GFP::unc-54UTR]; vxEx1211[Pmyo-3::mCherry::UNC54UTR]
JPS1664 vxIs591[vxIs591[ptph-1::GFP::unc-54UTR]; vxEx1211[Pmyo-3::mCherry::UNC54UTR]; ptl-1(ok621) III
JPS1667 vxIs591[vxIs591[ptph-1::GFP::unc-54UTR]; vxEx1213[Prab-3::ND18ApoE4::UNC54UTR + Pmyo-3::mCherry::UNC54UTR]
JPS1778 vxIs591[vxIs591[ptph-1::GFP::unc-54UTR]; vxEx1213[Prab-3::ND18ApoE4::UNC54UTR + Pmyo-3::mCherry::UNC54UTR]; ptl-1(ok621) III
JPS1779 vxIs591[vxIs591[ptph-1::GFP::unc-54UTR]; vxEx1213[Prab-3::ND18ApoE4::UNC54UTR + Pmyo-3::mCherry::UNC54UTR]; ptl-1(ok621) III
Figure 3
JPS1571 vxIs591[vxIs591[ptph-1::GFP::unc-54UTR]
JPS1828 vxEx1511[Pmec-17::EGL-1::egl-1UTR]
TU253 mec-4(u253) X
JPS1500 vxIs824[prab-3::ND18ApoE4::UNC54UTR, pmyo-2::mCherry::UNC54UTR]; ptl-1(pg73[PTL-1::mNeonGreen])
JPS1839 vxIs824[prab-3::ND18ApoE4::UNC54UTR, pmyo-2::mCherry::UNC54UTR]; ptl-1(pg73[PTL-1::mNeonGreen]); vxEx1511[Pmec-17::EGL-1::egl-1UTR]
JPS1709 vxIs824[prab-3::ND18ApoE4::UNC54UTR, pmyo-2::mCherry::UNC54UTR]; ptl-1(pg73[PTL-1::mNeonGreen]); mec-4(u253) X
Figure 4
JPS1571 vxIs591[vxIs591[ptph-1::GFP::unc-54UTR]
JPS1456 ptl-1(pg73[PTL-1::mNeonGreen]);vxEx1216[Pmec-17::ptl-1 sense and antisense CDS + Punc-122::RFP]
JPS1500 vxIs824[prab-3::ND18ApoE4::UNC54UTR, pmyo-2::mCherry::UNC54UTR]; ptl-1(pg73[PTL-1::mNeonGreen])
JPS1581 vxIs824[prab-3::ND18ApoE4::UNC54UTR, pmyo-2::mCherry::UNC54UTR]; ptl-1(pg73[PTL-1::mNeonGreen]); vxEx1216[Pmec-17::ptl-1 sense and antisense CDS + Punc-122::RFP]
JPS1582 vxIs824[prab-3::ND18ApoE4::UNC54UTR, pmyo-2::mCherry::UNC54UTR]; ptl-1(pg73[PTL-1::mNeonGreen]); vxEx1216[Pmec-17::ptl-1 sense and antisense CDS + Punc-122::RFP]
JPS1823 vxIs824[prab-3::ND18ApoE4::UNC54UTR, pmyo-2::mCherry::UNC54UTR]; ptl-1(syb8686 syb8597 pg73[LoxP::PTL-1::mNeonGreen::LoxP) III; hrtSi99[Pmec-17::Cre]
JPS1824 vxIs824[prab-3::ND18ApoE4::UNC54UTR, pmyo-2::mCherry::UNC54UTR]; ptl-1(syb8686 syb8597 pg73[LoxP::PTL-1::mNeonGreen::LoxP) III; hrtSi99[Pmec-17::Cre]
Generation of strains
Presence of the 1933 bp deletion in ptl-1 allele ok621 was confirmed by PCR using the following primers (written in 5’−3’ orientation):
Forward: CTGGAAATTTGTTGGGCAGT
Reverse: TGAACCGAAGCCTAAACCAG
We sought to independently confirm the effect of pan-neuronal APOE4 on egg laying as well as to test other behaviors with new extrachromosomal transgenic strains that carry a new reporter that minimized effects on multiple behaviors. We transformed wild-type N2 with 20 ng/μL of the APOE4 plasmid pPS90 (Sae-Lee et al., 2020) along with 10 ng/μL of the pCFJ104 mCherry body wall reporter (Frøkjaer-Jensen et al., 2008) to generate the new extrachromosomal strain JPS1453. In parallel, we transformed the ptl-1(ok621) mutant strain RB809 with the same DNA mixture yielding the APOE4;Δptl-1 strain JPS1455. We also generated control strain JPS1451 that carries the mCherry reporter alone.
To visualize the HSN axons, we used the Ptph-1::gfp reporter in strain JPS1571. This strain was crossed with JPS1451 and JPS1453 to yield the control and APOE4 strains JPS1666 and JPS1667, respectively. JPS1666 was then crossed with RB809 to yield the Ptph-1::gfp;Δptl-1 strain JPS1664. JPS1667 was crossed with RB809 to yield APOE4;Δptl-1, from which two independent isolates were obtained, JPS1778 and JPS1779, to partly control for genetic background.
Cell-specific gene knockdown
To knock down ptl-1 specifically in the touch receptor neurons (TRNs), we followed previous methods by Esposito et al. (See Fig. 1 of Esposito et al., 2007). The mec-17 promoter (1535 bp) was fused with either sense or antisense of ptl-1 coding region (820 bp) to generate separate DNA fragments using the following primers (written in 5’−3’ orientation):
A
Forward: GGCTTGAATAATCCCTATTATAGCC
Reverse: GAAATTACCTGAAATTTCGTGGGG
B
Forward: GAGAAAACGGCCAATTGACGC
Reverse: GGCTATAATAGGGATTATTCAAGCCGATCGAATCGTCTCACAACTG
C
Forward: GAGAAAACGGCCAATTGACGC
Reverse: CCCCACGAAATTTCAGGTAATTTCGATCGAATCGTCTCACAACTG
D
Forward: CGTCGCGCTAACAGTTCTAG
Reverse: CCAAAGGTTAACGCCAAATTTG
E
Forward: CGTCGCGCTAACAGTTCTAG
Reverse: CAAGTGAAAGCGTGGTCCGATC
Fragments D and E were mixed in equimolar amounts, along with a selectable RFP coelomocyte marker (Addgene Plasmid #8938), to a final concentration of 80 ng/μL. This DNA mix was transformed into the ptl-1::mNeonGreen strain GN655 (PTL-1::mNG; Krieg et al., 2017). The resultant strain, JPS1456, expresses ptl-1 dsRNA fragments in the TRNs. JPS1456 was crossed into JPS1500, which carries an integrated pan-neuronal APOE4 transgene, to yield two independent APOE4;TRNs::ptl-1 KD strains named JPS1581 and JPS1582.
Cell-specific gene knockout
To knock out ptl-1 in the TRNs, we commissioned SUNY Biotech to generate the strain PHX8686 which harbors LoxP sites flanking the ptl-1 genetic locus in the strain GN655. PHX8686 was crossed into MSB1167, a transgenic line in which Cre recombinase is expressed under control of the mec-17 promoter (Harterink et al., 2018). This yielded the TRNs::ptl-1 KO strain JPS1819. The presence of PTL-1::mNG in the TRNs was determined with epifluorescent microscopy. Lastly, JPS1819 was crossed into JPS1500 to yield APOE4;TRNs::ptl-1 KO, from which two independent isolates were obtained which are JPS1823 and JPS1824, to partly control for genetic background.
Cell-specific ablation
To genetically ablate the TRNs, we used the TRN-specific mec-17 promoter to express egl-1 in an extrachromosomal array in strain JPS1828 (Wu et al., 2025). This strain was crossed into JPS1500, a strain carrying an integrated pan-neuronal APOE4 transgene and a Punc-122::gfp reporter, to yield strain JPS1839. Additionally, we crossed JPS1500 into TU253, which carries mec-4 null mutation (O’Hagan et al., 2004), to yield strain APOE4;mec-4 JPS1709.
Sterilization
To inspect HSN neuron health without confounding effects of the bagging phenotype, worms were sterilized as previously described (Mitchell et al., 1979). In brief, seeded plates were pretreated with 600 mL of 0.8 mM 5’-fluorodeoxyuridine (FUdR; FUdR plates). Mid- to late-stage L4 stage worms were transferred onto FUDR plates and moved to fresh FUdR plates every 1–2 days as needed.
Behavioral assays
Behavioral assays were performed on either 60 mm (medium) or 35 mm (small) NGM plates, seeded or not with OP50 bacteria as described per assay. All assays were performed at room temperature (20° C) unless stated otherwise. Assays began with day 1 adults (D1), 24 hours after initial incubation on FUDR plates (if applicable), until a significant difference between control and APOE4 worms was observed. Completely immobile worms or those which escaped from the side of the plate were omitted from assays and final quantifications.
Gentle touch
Methods adapted from Chalfie and Sulston (1981). In brief, 20–30 worms were moved to medium NGM-OP50 plates. To test gentle touch sensitivity, an eyelash hair, affixed by its shaft to a pipette tip, was used to deliver mechanical stimuli to worms. Gentle strokes were applied to the posterior and anterior of the animal. Six paired anterior and posterior stimuli were applied, with an interstimulus interval of approximately 1.5 seconds to avoid habituation. Gentle touch sensitivity was quantified per worm as the fraction of responses to stimuli out of 12.
Harsh touch
Methods adapted from Li et al. (2011). In brief, 20–30 worms were moved to medium NGM-OP50 plates. To test harsh gentle touch sensitivity, a single prod was delivered to the mid-body using a platinum pick. Five technical replicates were performed per worm per strain. Harsh touch sensitivity was quantified per replicate as the proportion of responsive worms.
Pharyngeal pumping
Methods adapted from Avery and Shtonda (2003). In brief, 30–40 worms were first moved to medium NGM-OP50 plates. Worms were allowed to roam for at least 30 minutes. Individual animals were then tracked for 20 seconds and pharyngeal bulb contractions were manually tallied with a handheld click counter. Pumping rate was quantified per worm as pumps per minute.
Bagging
Methods adapted from Sae-Lee et al. (2020). In brief, at least 30–40 L4-stage worms were moved to medium NGM-OP50 plates. Worms were scored daily for the internal hatching or “bagging” phenotype based on presence or absence of several late-stage eggs and/or hatched larvae within the uterus; this is often accompanied by general lethargy plus distension of the worm midbody as previously described (Trent et al., 1983). Bagging worms were tallied and discarded while non-bagging worms were transferred to a fresh medium NGM-OP50 plate. Worms were scored in this fashion from D1 to D4 of adulthood. Bagging incidence was quantified per replicate as the cumulative percentage of bagging worms summed across D1–4 out of the original total.
Long-term locomotion
Methods adapted from Nordquist et al. (2018). Single worms were moved to the center of a small NGM plate uniformly seeded with OP50 such that the lawn covered the entirety of the NGM surface. Animals were incubated on these assay plates overnight at 22°C. Groups of at least 8 worms per genotype were tested on two separate days as also described by Nordquist et al. After 18 hours, the worms were removed from their plates and a transparent grid guide was used to determine the number of ~5 mm2 regions (32 in total) traversed on the assay plate. Regions encompassing parts of the NGM which split or pulled away from the plastic of the plate were omitted from the total. Long-term locomotion was quantified per worm as the proportion of traversed regions on the assay plate out of the total.
Short-term locomotion
Methods adapted from Topalidou et al. (2017). In brief, 8–10 worms were moved to the center of a medium NGM-OP50 plate which was uniformly seeded such that the bacterial lawn extended across the entire medium surface. After 10 minutes, worms were removed and their final positions marked on the plate. Groups of least 15 worms per genotype were tested on two separate days as previously described (Nordquist et al., 2018). The distance which they had radially dispersed was recorded. Short-term locomotion was quantified per worm as the distance (cm) from the center to final positions.
Swim-to-crawl gait transition
Methods adapted from Vidal-Gadea et al. (2011). Before testing, 25–40 worms were moved to a medium NGM plate and allowed to roam for ~15 minutes so excess OP50 could be removed. Meanwhile, assay plates were prepared by first drawing a 0.5 cm radius border on the plastic behind its center. Next, 10 uL of liquid NGM was pipetted to the very center of the assay plate surface. Worms were then picked to the center of the puddle on the assay plate and a 15-minute timer was started. As the puddle dried, swimming worms began to escape to the solid surface, transition to a crawling gait, and disperse away from the puddle. Gait transition was quantified per replicate as the proportion of worms which escaped and crawled past the 0.5 cm border in under 15 minutes.
Microscopy
Epifluorescent microscopy was performed on an Olympus IX51 inverted microscope with an Olympus UPlanFL N 40X/0.75 NA objective and X-Cite FIRE LED Illuminator (Excelitas Technologies Corp.). Images were captured using a Retiga 2000R CCD camera (QImaging) and QCapture Pro 6.0 software.
Worms were sterilized with FUDR as described above to prevent internally hatching larvae that may affect HSN morphology. Worms were then mounted on 2% agarose pads and immobilized using 50 mM sodium azide. All imaging per pad took place within 30 minutes of mounting.
The same fluorescence intensity settings were used to image all HSN soma and proximal axons via a GFP reporter. Importantly, imaging was restricted to worms which lay in a strictly lateral orientation (i.e., on the right or left side with minimal twisting about the long axis of the animal). This ensured both the soma and proximal axon of a given HSN neuron were in approximately the same focal plane. We determined the orientation of mounted worms using the fluorescent co-injection marker expressed in the strains which were imaged. This marker labels body wall and vulval musculature with mCherry, visualizable in the red channel. In single focal planes, the labeled vulval muscles either appear as an x-shape from a ventral viewpoint or a v-shape if the animal laid directly on its right or left side as described above (See also Fig. 1 of Collins and Koelle, 2020). Incidentally, the HSN GFP reporter also labels the VC4 and VC5 motor neurons, which are visible in the “v-shape” focal plane. These guides helped restrict our microscopy to animals in the desired orientation described above.
After imaging, morphometrics of HSN soma and proximal axons were calculated in ImageJ (Schneider et al., 2012). Positions of HSN soma were determined by measuring the lengths of the vector from the soma to the ventral nerve cord (HSN-VNC, met at a perpendicular) and the vector to the vulval area in between VCs4,5 (HSN-vulva). These measurements were also collapsed into a single metric as previously described (Asakura et al., 2007). This metric, represented by θ, is the angle in degrees between the HSN-vulva (hypotenuse) and HSN-VNC (adjacent) vectors (i.e., arccosine of their ratio).
HSN proximal axon lengths were calculated with the NeuronJ plugin for ImageJ using default settings (Meijering et al., 2004). Traces were made by following the proximal axon from anterior face of the HSN soma to the point at which the axon exits the focal plane ventrally in the vulval area. Ectopic sprouts from the soma or proximal axon were excluded from tracing. In the rare (<5% frequency) cases where ectopic sprouts were present on the proximal axon, the shortest ventral and vulval-directed course was traced for all strains imaged.
To capture images of PTL-1::mNG strains, an increased exposure time and intensity setting were required compared to HSN GFP reporter strains. Because some TRN processes span nearly the entire animal length, we chose to focus on soma to quantify PTL-1::mNG signal in TRNs::ptl-1 KD strain JPS1456 and control strain GN655 (See Fig. 3). Depending on animal orientation, bilateral TRN soma were identifiable based on the naturally asymmetric positioning of the AVM and PVM class neurons (Arnold et al., 2020). The VCs4,5 neurons were almost always visualizable regardless of orientation, making them ideal for internal controls. Fluorescence intensity was quantified in ImageJ in day 1 adults.
In the TRNs::ptl-1 KO strain JPS1819, LoxP-flanked ptl-1 is presumably excised by Cre recombinase whose expression is driven by the mec-17 promoter (Harterink et al., 2018). Although mec-17 expression begins embryonically in ALM and PLM class TRNs (Roux et al., 2023), residual PTL-1::mNG is still present by at least L4 stage of development; PTL-1::mNG is qualitatively fainter in those cells compared to like-aged control animals (GN655 strain). Thus, selection for animals with lacking PTL-1::mNG in PLM neurons was conducted in at least day 1 adults to avoid ambiguity. This selection strategy was also employed in the TRNs::EGL-1 strain JPS1828 to identify animals with genetically ablated TRNs.
Western blotting
Worms were washed twice with 5% sucrose and twice with 50 mM NaCl before being flash frozen with 5μl glass beads.
For worm lysis, 2% PMSF was added to 2x Laemmli buffer. This buffer was then added to the frozen samples to achieve a 1x concentration. Finally, 5% β-mercaptoethanol (BME) was added to the samples before bead beating, which was performed for 30 seconds. Samples were spun down through centrifugation for 2 minutes at 15,000 g. The samples were then boiled at 95℃ for 5 minutes. An additional 2-minute centrifugation was performed to clear debris prior to measuring protein concentration with Qubit Protein Assay Kit (Thermo Scientific, #Q33211).
20 μg of protein per sample was loaded on Invitrogen™ NuPAGE™ Bis-Tris Mini Protein Gels (4–12%, 1.0–1.5 mm, Invitrogen, #NP0335) and separated at 80–120 V in 1X MES SDS running buffer. Proteins were transferred onto a PVDF membrane (Thermo Scientific, #88518) at 30V for 1 hour.
Membranes were blocked in 1X TBS Tween 20 Buffer (Thermo Scientific, #28360) containing 5% milk. Flag tag and actin were probed with primary antibodies: Monoclonal ANTI-FLAG® M2 antibody produced in mouse (1:2,000, Sigma-Aldrich, #F3165) and Anti-Actin Mouse Monoclonal Antibody [clone: C4] (1:500, MP Biomedicals, #08691001), respectively. A secondary antibody, Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor™ 488 (1:4,000, Invitrogen, #A28175) was used after flag and actin probing. Finally, an Amersham™ Typhoon™ RGB was utilized for visualization.
Statistical analysis
All data are presented as the mean ± SEM. Plotted data points represent individuals or the average across a single replicate as indicated in figures. Total sample size (N) is indicated in figure legends. Behavioral assays and microscopy scoring were performed blind to genotype. χ2 tests of independence were performed for statistical comparisons of gait transition (escaped vs. not escaped), bagging (yes vs. no), and HSN axon scoring (normal vs. abnormal). One-tailed Student t-tests were performed for statistical comparisons of touch, pumping, locomotion behaviors, HSN axon length, HSN soma position, and fluorescence intensity.
Supplementary Material
Acknowledgements
We would like to thank Drs. Miriam B. Goodman and Michael Krieg for sharing strains GN655 and MSB1167, respectively, and SUNY Biotech for engineering PHX8686. Some strains were also provided by the Caenorhabditis Genetics Center, which is funded by the NIH Center for Research Resources. We also thank Chelsea Webber for constructive feedback on manuscript drafts.
Funding
This work was funded in part by the NIH (R01GM122463, RF1AG057355, R21OD032463 - J.T.P.) Waggoner Fellowship for Alcohol Research (J.T.P.), David and Ellen Berman Fellowships for Huntington’s Research (J.T.P.), George and Karen Casey (J.T.P.), Pine Family Foundation (J.T.P.), CNS Catalyst Grant (J.T.P. and E.S.C.), National Institutes of Health (NIH) NIGMS (R35GM138340) (E.S.C.), Welch Foundation (F-2133-20230405) (E.S.C.), and F.M. Jones and H.L. Bruce Graduate Fellowship (Z.W.).
Footnotes
Competing interests
The authors declare no competing or financial interests.
Data availability
All relevant data can be found either within the article or in its supplementary information section. Raw data is available upon request.
References
- Andrews-Zwilling Y., Bien-Ly N., Xu Q., Li G., Bernardo A., Yoon S. Y., Zwilling D., Yan T. X., Chen L., and Huang Y. (2010). Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience, 30(41), 13707–13717. 10.1523/JNEUROSCI.4040-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino Nunez W., Combs B., Gamblin T. C., and Ackley B. D. (2022). Age-dependent accumulation of tau aggregation in Caenorhabditis elegans. Frontiers in aging, 3, 928574. 10.3389/fragi.2022.928574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold M. L., Cooper J., Grant B. D., & Driscoll M. (2020). Quantitative Approaches for Scoring in vivo Neuronal Aggregate and Organelle Extrusion in Large Exopher Vesicles in C. elegans. Journal of visualized experiments : JoVE, (163), 10.3791/61368. https://doi.org/10.3791/61368 [DOI] [PMC free article] [PubMed]
- Asakura T., Ogura K., and Goshima Y. (2007). UNC-6 expression by the vulval precursor cells of Caenorhabditis elegans is required for the complex axon guidance of the HSN neurons. Developmental biology, 304(2), 800–810. 10.1016/j.ydbio.2007.01.028 [DOI] [PubMed] [Google Scholar]
- Avery L., and Shtonda B. B. (2003). Food transport in the C. elegans pharynx. The Journal of experimental biology, 206(Pt 14), 2441–2457. 10.1242/jeb.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L., and You Y. J. (2012). C. elegans feeding. WormBook : the online review of C. elegans biology, 1–23. 10.1895/wormbook.1.150.1 [DOI] [PMC free article] [PubMed]
- Baskoylu S. N., Yersak J., O’Hern P., Grosser S., Simon J., Kim S., Schuch K., Dimitriadi M., Yanagi K. S., Lins J., and Hart A. C. (2018). Single copy/knock-in models of ALS SOD1 in C. elegans suggest loss and gain of function have different contributions to cholinergic and glutamatergic neurodegeneration. PLoS genetics, 14(10), e1007682. 10.1371/journal.pgen.1007682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbow S. J., Strovas T. J., Darvas M., Saxton A., and Kraemer B. C. (2020). Synergistic toxicity between tau and amyloid drives neuronal dysfunction and neurodegeneration in transgenic C. elegans. Human molecular genetics, 29(3), 495–505. 10.1093/hmg/ddz319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing W. C., and Mufson E. J. (1995). Apolipoprotein E immunoreactivity within neurofibrillary tangles: relationship to Tau and PHF in Alzheimer’s disease. Experimental neurology, 132(2), 162–171. 10.1016/0014-4886(95)90021-7 [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Y., Strojwas M. H., Cohen M. S., Saunders A. M., Pericak-Vance M. A., Mazziotta J. C., and Small G. W. (2000). Patterns of brain activation in people at risk for Alzheimer’s disease. The New England journal of medicine, 343(7), 450–456. 10.1056/NEJM200008173430701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., and Braak E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica, 82(4), 239–259. 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- Braak H., & Del Tredici K. (2011). Alzheimer’s pathogenesis: is there neuron-to-neuron propagation?. Acta neuropathologica, 121(5), 589–595. 10.1007/s00401-011-0825-z [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics, 77(1), 71–94. 10.1093/genetics/77.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht W. J., Harris F. M., Chang S., Tesseur I., Yu G. Q., Xu Q., Dee Fish J., Wyss-Coray T., Buttini M., Mucke L., Mahley R. W., and Huang Y. (2004). Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience, 24(10), 2527–2534. 10.1523/JNEUROSCI.4315-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butner K. A., and Kirschner M. W. (1991). Tau protein binds to microtubules through a flexible array of distributed weak sites. The Journal of cell biology, 115(3), 717–730. 10.1083/jcb.115.3.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., and Sulston J. (1981). Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Developmental biology, 82(2), 358–370. 10.1016/0012-1606(81)90459-0 [DOI] [PubMed] [Google Scholar]
- Chalfie M., and Thomson J. N. (1979). Organization of neuronal microtubules in the nematode Caenorhabditis elegans. The Journal of cell biology, 82(1), 278–289. 10.1083/jcb.82.1.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., and Thomson J. N. (1982). Structural and functional diversity in the neuronal microtubules of Caenorhabditis elegans. The Journal of cell biology, 93(1), 15–23. 10.1083/jcb.93.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Sulston J. E., White J. G., Southgate E., Thomson J. N., and Brenner S. (1985). The neural circuit for touch sensitivity in Caenorhabditis elegans. The Journal of neuroscience : the official journal of the Society for Neuroscience, 5(4), 956–964. 10.1523/JNEUROSCI.05-04-00956.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. J., Chronis N., Karow D. S., Marletta M. A., and Bargmann C. I. (2006). A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS biology, 4(9), e274. 10.1371/journal.pbio.0040274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F., Bolmont T., Crowther R. A., Abramowski D., Frank S., Probst A., Fraser G., Stalder A. K., Beibel M., Staufenbiel M., Jucker M., Goedert M., and Tolnay M. (2009). Transmission and spreading of tauopathy in transgenic mouse brain. Nature cell biology, 11(7), 909–913. 10.1038/ncb1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakley S., Ritchie F. K., Galbraith K. M., and Hilliard M. A. (2020). Epidermal control of axonal attachment via β-spectrin and the GTPase-activating protein TBC-10 prevents axonal degeneration. Nature communications, 11(1), 133. 10.1038/s41467-019-13795-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblath E. J., Li H. L., Changolkar L., Zhang B., Brown H. J., Gathagan R. J., Olufemi M. F., Trojanowski J. Q., Bassett D. S., Lee V. M. Y., and Henderson M. X. (2021). Computational modeling of tau pathology spread reveals patterns of regional vulnerability and the impact of a genetic risk factor. Science advances, 7(24), eabg6677. 10.1126/sciadv.abg6677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Biel N., Theunis C., Borghgraef P., Lechat B., Devijver H., Maurin H., and Van Leuven F. (2014). Phosphorylation of protein Tau by GSK3β prolongs survival of bigenic Tau.P301L×GSK3β mice by delaying brainstem tauopathy. Neurobiology of disease, 67, 119–132. 10.1016/j.nbd.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Daigle I., and Li C. (1993). apl-1, a Caenorhabditis elegans gene encoding a protein related to the human beta-amyloid protein precursor. Proceedings of the National Academy of Sciences of the United States of America, 90(24), 12045–12049. 10.1073/pnas.90.24.12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. A., Inman C. E., Wargel Z. M., Dube U., Freeberg B. M., Galluppi A., Haines J. N., Dhavale D. D., Miller R., Choudhury F. A., Sullivan P. M., Cruchaga C., Perlmutter J. S., Ulrich J. D., Benitez B. A., Kotzbauer P. T., and Holtzman D. M. (2020). APOE genotype regulates pathology and disease progression in synucleinopathy. Science translational medicine, 12(529), eaay3069. 10.1126/scitranslmed.aay3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Calignon A., Polydoro M., Suárez-Calvet M., William C., Adamowicz D. H., Kopeikina K. J., Pitstick R., Sahara N., Ashe K. H., Carlson G. A., Spires-Jones T. L., and Hyman B. T. (2012). Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron, 73(4), 685–697. 10.1016/j.neuron.2011.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVos S. L., Miller R. L., Schoch K. M., Holmes B. B., Kebodeaux C. S., Wegener A. J., Chen G., Shen T., Tran H., Nichols B., Zanardi T. A., Kordasiewicz H. B., Swayze E. E., Bennett C. F., Diamond M. I., and Miller T. M. (2017). Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Science translational medicine, 9(374), eaag0481. 10.1126/scitranslmed.aag0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichel K., Uenaka T., Belapurkar V., Lu R., Cheng S., Pak J. S., Taylor C. A., Südhof T. C., Malenka R., Wernig M., Özkan E., Perrais D., and Shen K. (2022). Endocytosis in the axon initial segment maintains neuronal polarity. Nature, 609(7925), 128–135. 10.1038/s41586-022-05074-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G., Di Schiavi E., Bergamasco C., and Bazzicalupo P. (2007). Efficient and cell specific knock-down of gene function in targeted C. elegans neurons. Gene, 395(1–2), 170–176. 10.1016/j.gene.2007.03.002 [DOI] [PubMed] [Google Scholar]
- Faber P. W., Alter J. R., MacDonald M. E., and Hart A. C. (1999). Polyglutamine-mediated dysfunction and apoptotic death of a Caenorhabditis elegans sensory neuron. Proceedings of the National Academy of Sciences of the United States of America, 96(1), 179–184. 10.1073/pnas.96.1.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros C., Pir G. J., Biernat J., Koushika S. P., Mandelkow E., Mandelkow E. M., Schmidt E., and Baumeister R. (2012). Inhibition of tau aggregation in a novel Caenorhabditis elegans model of tauopathy mitigates proteotoxicity. Human molecular genetics, 21(16), 3587–3603. 10.1093/hmg/dds190 [DOI] [PubMed] [Google Scholar]
- Fiford C. M., Ridgway G. R., Cash D. M., Modat M., Nicholas J., Manning E. N., Malone I. B., Biessels G. J., Ourselin S., Carmichael O. T., Cardoso M. J., Barnes J., and Alzheimer’s Disease Neuroimaging Initiative (2018). Patterns of progressive atrophy vary with age in Alzheimer’s disease patients. Neurobiology of aging, 63, 22–32. 10.1016/j.neurobiolaging.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell S. W., Raizen D. M., and You Y. J. (2020). Behavioral States. Genetics, 216(2), 315–332. 10.1534/genetics.120.303539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortea J., Pegueroles J., Alcolea D., Belbin O., Dols-Icardo O., Vaqué-Alcázar L., Videla L., Gispert J. D., Suárez-Calvet M., Johnson S. C., Sperling R., Bejanin A., Lleó A., and Montal V. (2024). APOE4 homozygozity represents a distinct genetic form of Alzheimer’s disease. Nature medicine, 30(5), 1284–1291. 10.1038/s41591-024-02931-w [DOI] [PubMed] [Google Scholar]
- Friedhoff P., Schneider A., Mandelkow E. M., and Mandelkow E. (1998). Rapid assembly of Alzheimer-like paired helical filaments from microtubule-associated protein tau monitored by fluorescence in solution. Biochemistry, 37(28), 10223–10230. 10.1021/bi980537d [DOI] [PubMed] [Google Scholar]
- Friedhoff P., von Bergen M., Mandelkow E. M., Davies P., and Mandelkow E. (1998). A nucleated assembly mechanism of Alzheimer paired helical filaments. Proceedings of the National Academy of Sciences of the United States of America, 95(26), 15712–15717. 10.1073/pnas.95.26.15712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjaer-Jensen C., Davis M. W., Hopkins C. E., Newman B. J., Thummel J. M., Olesen S. P., Grunnet M., and Jorgensen E. M. (2008). Single-copy insertion of transgenes in Caenorhabditis elegans. Nature genetics, 40(11), 1375–1383. 10.1038/ng.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B., and Diamond M. I. (2010). Prion-like mechanisms in neurodegenerative diseases. Nature reviews. Neuroscience, 11(3), 155–159. 10.1038/nrn2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H., Hussaini S. A., Wegmann S., Profaci C., Daniels J. D., Herman M., Emrani S., Figueroa H. Y., Hyman B. T., Davies P., and Duff K. E. (2016). 3D Visualization of the Temporal and Spatial Spread of Tau Pathology Reveals Extensive Sites of Tau Accumulation Associated with Neuronal Loss and Recognition Memory Deficit in Aged Tau Transgenic Mice. PloS one, 11(7), e0159463. 10.1371/journal.pone.0159463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Guan S. A., Fouad A. D., Meng J., Kawano T., Huang Y. C., Li Y., Alcaire S., Hung W., Lu Y., Qi Y. B., Jin Y., Alkema M., Fang-Yen C., and Zhen M. (2018). Excitatory motor neurons are local oscillators for backward locomotion. eLife, 7, e29915. 10.7554/eLife.29915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga G., Desai C., and Horvitz H. R. (1993). Cell interactions control the direction of outgrowth, branching and fasciculation of the HSN axons of Caenorhabditis elegans. Development (Cambridge, England), 117(3), 1071–1087. 10.1242/dev.117.3.1071 [DOI] [PubMed] [Google Scholar]
- Ghoshal N., García-Sierra F., Wuu J., Leurgans S., Bennett D. A., Berry R. W., and Binder L. I. (2002). Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer’s disease. Experimental neurology, 177(2), 475–493. 10.1006/exnr.2002.8014 [DOI] [PubMed] [Google Scholar]
- Goedert M., Baur C. P., Ahringer J., Jakes R., Hasegawa M., Spillantini M. G., Smith M. J., and Hill F. (1996). PTL-1, a microtubule-associated protein with tau-like repeats from the nematode Caenorhabditis elegans. Journal of cell science, 109 (Pt 11), 2661–2672. 10.1242/jcs.109.11.2661 [DOI] [PubMed] [Google Scholar]
- Gordon P., Hingula L., Krasny M. L., Swienckowski J. L., Pokrywka N. J., and Raley-Susman K. M. (2008). The invertebrate microtubule-associated protein PTL-1 functions in mechanosensation and development in Caenorhabditis elegans. Development genes and evolution, 218(10), 541–551. 10.1007/s00427-008-0250-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., and Wisniewski H. M. (1986). Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. The Journal of biological chemistry, 261(13), 6084–6089. [PubMed] [Google Scholar]
- Harterink M., Edwards S. L., de Haan B., Yau K. W., van den Heuvel S., Kapitein L. C., Miller K. G., and Hoogenraad C. C. (2018). Local microtubule organization promotes cargo transport in C. elegans dendrites. Journal of cell science, 131(20), jcs223107. 10.1242/jcs.223107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashi Y., Kotani S., and Adachi T. (2016). A nematode microtubule-associated protein, PTL-1, closely resembles its mammalian counterparts in overall molecular architecture. Bioscience, Biotechnology, and Biochemistry, 80(6), 1107–1113. 10.1080/09168451.2016.1141038 [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. (2003). Worms, life, and death (Nobel lecture). Chembiochem : a European journal of chemical biology, 4(8), 697–711. 10.1002/cbic.200300614 [DOI] [PubMed] [Google Scholar]
- Huang Y., Liu X. Q., Wyss-Coray T., Brecht W. J., Sanan D. A., and Mahley R. W. (2001). Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proceedings of the National Academy of Sciences of the United States of America, 98(15), 8838–8843. 10.1073/pnas.151254698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba M., Guo J. L., McBride J. D., Zhang B., Trojanowski J. Q., and Lee V. M. (2013). Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. The Journal of neuroscience : the official journal of the Society for Neuroscience, 33(3), 1024–1037. 10.1523/JNEUROSCI.2642-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo E. J., and Beer R. D. (2018). From head to tail: a neuromechanical model of forward locomotion in Caenorhabditis elegans. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 373(1758), 20170374. 10.1098/rstb.2017.0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeganathan S., Hascher A., Chinnathambi S., Biernat J., Mandelkow E. M., and Mandelkow E. (2008). Proline-directed pseudo-phosphorylation at AT8 and PHF1 epitopes induces a compaction of the paperclip folding of Tau and generates a pathological (MC-1) conformation. The Journal of biological chemistry, 283(46), 32066–32076. 10.1074/jbc.M805300200 [DOI] [PubMed] [Google Scholar]
- Jeganathan S., von Bergen M., Brutlach H., Steinhoff H. J., and Mandelkow E. (2006). Global hairpin folding of tau in solution. Biochemistry, 45(7), 2283–2293. 10.1021/bi0521543 [DOI] [PubMed] [Google Scholar]
- Jiang W. I., Cao Y., Xue Y., Ji Y., Winer B. Y., Chandra R., Zhang X. F., Zhang M., Singhal N. S., Pierce J. T., Chen S., and Ma D. K. (2025). Suppressing APOE4-induced neural pathologies by targeting the VHL-HIF axis. Proceedings of the National Academy of Sciences of the United States of America, 122(5), e2417515122. 10.1073/pnas.2417515122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kfoury N., Holmes B. B., Jiang H., Holtzman D. M., and Diamond M. I. (2012). Trans-cellular propagation of Tau aggregation by fibrillar species. The Journal of biological chemistry, 287(23), 19440–19451. 10.1074/jbc.M112.346072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köpke E., Tung Y. C., Shaikh S., Alonso A. C., Iqbal K., and Grundke-Iqbal I. (1993). Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. The Journal of biological chemistry, 268(32), 24374–24384. [PubMed] [Google Scholar]
- Koriath C., Lashley T., Taylor W., Druyeh R., Dimitriadis A., Denning N., Williams J., Warren J. D., Fox N. C., Schott J. M., Rowe J. B., Collinge J., Rohrer J. D., and Mead S. (2019). ApoE4 lowers age at onset in patients with frontotemporal dementia and tauopathy independent of amyloid-β copathology. Alzheimer’s and dementia (Amsterdam, Netherlands), 11, 277–280. 10.1016/j.dadm.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., and Lee G. (1988). Epitopes that span the tau molecule are shared with paired helical filaments. Neuron, 1(9), 817–825. 10.1016/0896-6273(88)90129-8 [DOI] [PubMed] [Google Scholar]
- Koutsodendris N., Blumenfeld J., Agrawal A., Traglia M., Grone B., Zilberter M., Yip O., Rao A., Nelson M. R., Hao Y., Thomas R., Yoon S. Y., Arriola P., and Huang Y. (2023). Neuronal APOE4 removal protects against tau-mediated gliosis, neurodegeneration and myelin deficits. Nature aging, 3(3), 275–296. 10.1038/s43587-023-00368-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall N. W., and Kosik K. S. (1987). Axonal disruption and aberrant localization of tau protein characterize the neuropil pathology of Alzheimer’s disease. Annals of neurology, 22(5), 639–643. 10.1002/ana.410220514 [DOI] [PubMed] [Google Scholar]
- Kraemer B. C., Zhang B., Leverenz J. B., Thomas J. H., Trojanowski J. Q., and Schellenberg G. D. (2003). Neurodegeneration and defective neurotransmission in a Caenorhabditis elegans model of tauopathy. Proceedings of the National Academy of Sciences of the United States of America, 100(17), 9980–9985. 10.1073/pnas.1533448100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M., Stühmer J., Cueva J. G., Fetter R., Spilker K., Cremers D., Shen K., Dunn A. R., and Goodman M. B. (2017). Genetic defects in β-spectrin and tau sensitize C. elegans axons to movement-induced damage via torque-tension coupling. eLife, 6, e20172. 10.7554/eLife.20172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen F. K., Baksh R. A., McGlinchey E., Langballe E. M., Benejam B., Beresford-Webb J., McCarron M., Coppus A., Falquero S., Fortea J., Levin J., Loosli S. V., Mark R., Rebillat A. S., Zaman S., and Strydom A. (2024). Age of Alzheimer’s disease diagnosis in people with Down syndrome and associated factors: Results from the Horizon 21 European Down syndrome consortium. Alzheimer’s and dementia : the journal of the Alzheimer’s Association, 20(5), 3270–3280. 10.1002/alz.13779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Viqar F., Zimmerman M. E., Narkhede A., Tosto G., Benzinger T. L., Marcus D. S., Fagan A. M., Goate A., Fox N. C., Cairns N. J., Holtzman D. M., Buckles V., Ghetti B., McDade E., Martins R. N., Saykin A. J., Masters C. L., Ringman J. M., Ryan N. S., … Dominantly Inherited Alzheimer Network (2016). White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Annals of neurology, 79(6), 929–939. 10.1002/ana.24647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., and Trojanowski J. Q. (1992). The disordered neuronal cytoskeleton in Alzheimer’s disease. Current opinion in neurobiology, 2(5), 653–656. 10.1016/0959-4388(92)90034-i [DOI] [PubMed] [Google Scholar]
- Leroy K., Yilmaz Z., and Brion J. P. (2007). Increased level of active GSK-3beta in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathology and applied neurobiology, 33(1), 43–55. 10.1111/j.1365-2990.2006.00795.x [DOI] [PubMed] [Google Scholar]
- Li W., Kang L., Piggott B. J., Feng Z., and Xu X. Z. (2011). The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans. Nature communications, 2, 315. 10.1038/ncomms1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Silva T. B., Bahia V. S., Carvalho V. A., Guimarães H. C., Caramelli P., Balthazar M., Damasceno B., Bottino C. M. C., Brucki S. M. D., Nitrini R., and Yassuda M. S. (2013). Functional profile of patients with behavioral variant frontotemporal dementia (bvFTD) compared to patients with Alzheimer’s disease and normal controls. Dementia and neuropsychologia, 7(1), 96–103. 10.1590/S1980-57642013DN70100015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. C., Nana A. L., Hepker M., Hwang J. L., Gaus S. E., Spina S., Cosme C. G., Gan L., Grinberg L. T., Geschwind D. H., Coppola G., Rosen H. J., Miller B. L., and Seeley W. W. (2019). Preferential tau aggregation in von Economo neurons and fork cells in frontotemporal lobar degeneration with specific MAPT variants. Acta neuropathologica communications, 7(1), 159. 10.1186/s40478-019-0809-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Drouet V., Wu J. W., Witter M. P., Small S. A., Clelland C., and Duff K. (2012). Trans-synaptic spread of tau pathology in vivo. PloS one, 7(2), e31302. 10.1371/journal.pone.0031302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch K. A., Qi Y. B., Takayanagi-Kiya S., Jin Y., and Cherra S. J. 3rd (2017). Novel Mutations in Synaptic Transmission Genes Suppress Neuronal Hyperexcitation in Caenorhabditis elegans. G3 (Bethesda, Md.), 7(7), 2055–2063. 10.1534/g3.117.042598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott J. B., Aamodt S., and Aamodt E. (1996). ptl-1, a Caenorhabditis elegans gene whose products are homologous to the tau microtubule-associated proteins. Biochemistry, 35(29), 9415–9423. 10.1021/bi952646n [DOI] [PubMed] [Google Scholar]
- Meijering E., Jacob M., Sarria J. C., Steiner P., Hirling H., and Unser M. (2004). Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry. Part A : the journal of the International Society for Analytical Cytology, 58(2), 167–176. 10.1002/cyto.a.20022 [DOI] [PubMed] [Google Scholar]
- Meisl G., Hidari E., Allinson K., Rittman T., DeVos S. L., Sanchez J. S., Xu C. K., Duff K. E., Johnson K. A., Rowe J. B., Hyman B. T., Knowles T. P. J., and Klenerman D. (2021). In vivo rate-determining steps of tau seed accumulation in Alzheimer’s disease. Science advances, 7(44), eabh1448. 10.1126/sciadv.abh1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Alfonso A., Nguyen M., Crowell J. A., Johnson C. D., and Rand J. B. (1996). A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proceedings of the National Academy of Sciences of the United States of America, 93(22), 12593–12598. 10.1073/pnas.93.22.12593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. H., Stiles J. W., Santelli J., and Sanadi D. R. (1979). Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. Journal of gerontology, 34(1), 28–36. 10.1093/geronj/34.1.28 [DOI] [PubMed] [Google Scholar]
- Mizumoto K., and Shen K. (2013). Interaxonal interaction defines tiled presynaptic innervation in C. elegans. Neuron, 77(4), 655–666. 10.1016/j.neuron.2012.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocanu M. M., Nissen A., Eckermann K., Khlistunova I., Biernat J., Drexler D., Petrova O., Schönig K., Bujard H., Mandelkow E., Zhou L., Rune G., and Mandelkow E. M. (2008). The potential for beta-structure in the repeat domain of tau protein determines aggregation, synaptic decay, neuronal loss, and coassembly with endogenous Tau in inducible mouse models of tauopathy. The Journal of neuroscience : the official journal of the Society for Neuroscience, 28(3), 737–748. 10.1523/JNEUROSCI.2824-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale C., Barzago M. M., and Diomede L. (2020). Caenorhabditis elegans Models to Investigate the Mechanisms Underlying Tau Toxicity in Tauopathies. Brain sciences, 10(11), 838. 10.3390/brainsci10110838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoletopoulou V., and Tavernarakis N. (2014). Necrotic cell death in Caenorhabditis elegans. Methods in enzymology, 545, 127–155. 10.1016/B978-0-12-801430-1.00006-8 [DOI] [PubMed] [Google Scholar]
- Nordquist S. K., Smith S. R., and Pierce J. T. (2018). Systematic Functional Characterization of Human 21st Chromosome Orthologs in Caenorhabditis elegans. G3 (Bethesda, Md.), 8(3), 967–979. 10.1534/g3.118.200019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuriel T., Angulo S. L., Khan U., Ashok A., Chen Q., Figueroa H. Y., Emrani S., Liu L., Herman M., Barrett G., Savage V., Buitrago L., Cepeda-Prado E., Fung C., Goldberg E., Gross S. S., Hussaini S. A., Moreno H., Small S. A., and Duff K. E. (2017). Neuronal hyperactivity due to loss of inhibitory tone in APOE4 mice lacking Alzheimer’s disease-like pathology. Nature communications, 8(1), 1464. 10.1038/s41467-017-01444-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley S. S., Maina M. B., Marshall K. E., Al-Hilaly Y. K., Harrington C. R., Wischik C. M., and Serpell L. C. (2020). Tau Filament Self-Assembly and Structure: Tau as a Therapeutic Target. Frontiers in neurology, 11, 590754. 10.3389/fneur.2020.590754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hagan R., Chalfie M., and Goodman M. B. (2005). The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nature neuroscience, 8(1), 43–50. 10.1038/nn1362 [DOI] [PubMed] [Google Scholar]
- Rietdijk C. D., Perez-Pardo P., Garssen J., van Wezel R. J., and Kraneveld A. D. (2017). Exploring Braak’s Hypothesis of Parkinson’s Disease. Frontiers in neurology, 8, 37. 10.3389/fneur.2017.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson E. D., Scearce-Levie K., Palop J. J., Yan F., Cheng I. H., Wu T., Gerstein H., Yu G. Q., and Mucke L. (2007). Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science (New York, N.Y.), 316(5825), 750–754. 10.1126/science.1141736 [DOI] [PubMed] [Google Scholar]
- Robinson J. L., Lee E. B., Xie S. X., Rennert L., Suh E., Bredenberg C., Caswell C., Van Deerlin V. M., Yan N., Yousef A., Hurtig H. I., Siderowf A., Grossman M., McMillan C. T., Miller B., Duda J. E., Irwin D. J., Wolk D., Elman L., McCluskey L., … Trojanowski J. Q. (2018). Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain : a journal of neurology, 141(7), 2181–2193. 10.1093/brain/awy146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn T. T., Catlin L. W., Coonse K. G., and Habig J. W. (2012). Identification of an amino-terminal fragment of apolipoprotein E4 that localizes to neurofibrillary tangles of the Alzheimer’s disease brain. Brain research, 1475, 106–115. 10.1016/j.brainres.2012.08.003 [DOI] [PubMed] [Google Scholar]
- Rondina J. M., Ferreira L. K., de Souza Duran F. L., Kubo R., Ono C. R., Leite C. C., Smid J., Nitrini R., Buchpiguel C. A., and Busatto G. F. (2017). Selecting the most relevant brain regions to discriminate Alzheimer’s disease patients from healthy controls using multiple kernel learning: A comparison across functional and structural imaging modalities and atlases. NeuroImage. Clinical, 17, 628–641. 10.1016/j.nicl.2017.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H. J., Allison S. C., Schauer G. F., Gorno-Tempini M. L., Weiner M. W., and Miller B. L. (2005). Neuroanatomical correlates of behavioural disorders in dementia. Brain : a journal of neurology, 128(Pt 11), 2612–2625. 10.1093/brain/awh628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A. E., Yuan H., Podshivalova K., Hendrickson D., Kerr R., Kenyon C., and Kelley D. (2023). Individual cell types in C. elegans age differently and activate distinct cell-protective responses. Cell reports, 42(8), 112902. 10.1016/j.celrep.2023.112902 [DOI] [PubMed] [Google Scholar]
- Ryan N. S., and Rossor M. N. (2010). Correlating familial Alzheimer’s disease gene mutations with clinical phenotype. Biomarkers in medicine, 4(1), 99–112. 10.2217/bmm.09.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sae-Lee W., Scott L. L., Brose L., Encarnacion A. J., Shi T., Kore P., Oyibo L. O., Ye C., Rozmiarek S. K., and Pierce J. T. (2020). APP-Induced Patterned Neurodegeneration Is Exacerbated by APOE4 in Caenorhabditis elegans. G3 (Bethesda, Md.), 10(8), 2851–2861. 10.1534/g3.120.401486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfeliu-Cerdán N., Català-Castro F., Mateos B., Garcia-Cabau C., Ribera M., Ruider I., Porta-de-la-Riva M., Canals-Calderón A., Wieser S., Salvatella X., and Krieg M. (2023). A MEC-2/stomatin condensate liquid-to-solid phase transition controls neuronal mechanotransduction during touch sensing. Nature cell biology, 25(11), 1590–1599. 10.1038/s41556-023-01247-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarella R. A., Skiniotis G., Goldie K. N., Tittmann P., Gross H., Mandelkow E. M., Mandelkow E., and Hoenger A. (2004). Surface-decoration of microtubules by human tau. Journal of molecular biology, 339(3), 539–553. 10.1016/j.jmb.2004.04.008 [DOI] [PubMed] [Google Scholar]
- Scahill R. I., Schott J. M., Stevens J. M., Rossor M. N., and Fox N. C. (2002). Mapping the evolution of regional atrophy in Alzheimer’s disease: unbiased analysis of fluid-registered serial MRI. Proceedings of the National Academy of Sciences of the United States of America, 99(7), 4703–4707. 10.1073/pnas.052587399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer W. R. (2005). Egg-laying. WormBook : the online review of C. elegans biology, 1–7. 10.1895/wormbook.1.38.1 [DOI] [PMC free article] [PubMed]
- Scharf A., Pohl F., Egan B. M., Kocsisova Z., and Kornfeld K. (2021). Reproductive Aging in Caenorhabditis elegans: From Molecules to Ecology. Frontiers in cell and developmental biology, 9, 718522. 10.3389/fcell.2021.718522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S., and Eliceiri K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9(7), 671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D. O., O’Donnell D., Jain N., Ulrich J. D., Herz J., Li Y., Lemieux M., Cheng J., Hu H., Serrano J. R., Bao X., Franke E., Karlsson M., Meier M., Deng S., Desai C., Dodiya H., Lelwala-Guruge J., Handley S. A., Kipnis J., … Holtzman D. M. (2023). ApoE isoform- and microbiota-dependent progression of neurodegeneration in a mouse model of tauopathy. Science (New York, N.Y.), 379(6628), eadd1236. 10.1126/science.add1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A., Das S., and Hyman B. T. (2021). APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. The Lancet. Neurology, 20(1), 68–80. 10.1016/S1474-4422(20)30412-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Yamada K., Liddelow S. A., Smith S. T., Zhao L., Luo W., Tsai R. M., Spina S., Grinberg L. T., Rojas J. C., Gallardo G., Wang K., Roh J., Robinson G., Finn M. B., Jiang H., Sullivan P. M., Baufeld C., Wood M. W., Sutphen C., … Holtzman D. M. (2017). ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature, 549(7673), 523–527. 10.1038/nature24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spittaels K., Van den Haute C., Van Dorpe J., Bruynseels K., Vandezande K., Laenen I., Geerts H., Mercken M., Sciot R., Van Lommel A., Loos R., and Van Leuven F. (1999). Prominent axonopathy in the brain and spinal cord of transgenic mice overexpressing four-repeat human tau protein. The American journal of pathology, 155(6), 2153–2165. 10.1016/S0002-9440(10)65533-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steciuk M., Cheong M., Waite C., You Y. J., and Avery L. (2014). Regulation of synaptic transmission at the Caenorhabditis elegans M4 neuromuscular junction by an antagonistic relationship between two calcium channels. G3 (Bethesda, Md.), 4(12), 2535–2543. 10.1534/g3.114.014308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang K. H., Golde T. E., and Giasson B. I. (2019). MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Laboratory investigation; a journal of technical methods and pathology, 99(7), 912–928. 10.1038/s41374-019-0197-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sündermann F., Fernandez M. P., and Morgan R. O. (2016). An evolutionary roadmap to the microtubule-associated protein MAP Tau. BMC genomics, 17, 264. 10.1186/s12864-016-2590-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. R., Santpere G., Weinreb A., Barrett A., Reilly M. B., Xu C., Varol E., Oikonomou P., Glenwinkel L., McWhirter R., Poff A., Basavaraju M., Rafi I., Yemini E., Cook S. J., Abrams A., Vidal B., Cros C., Tavazoie S., Sestan N., … Miller D. M. 3rd (2021). Molecular topography of an entire nervous system. Cell, 184(16), 4329–4347.e23. 10.1016/j.cell.2021.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therriault J., Benedet A. L., Pascoal T. A., Mathotaarachchi S., Chamoun M., Savard M., Thomas E., Kang M. S., Lussier F., Tissot C., Parsons M., Qureshi M. N. I., Vitali P., Massarweh G., Soucy J. P., Rej S., Saha-Chaudhuri P., Gauthier S., and Rosa-Neto P. (2020). Association of Apolipoprotein E ε4 With Medial Temporal Tau Independent of Amyloid-β. JAMA neurology, 77(4), 470–479. 10.1001/jamaneurol.2019.4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalidou I., Chen P. A., Cooper K., Watanabe S., Jorgensen E. M., and Ailion M. (2017). The NCA-1 and NCA-2 Ion Channels Function Downstream of Gq and Rho To Regulate Locomotion in Caenorhabditis elegans. Genetics, 206(1), 265–282. 10.1534/genetics.116.198820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent C., Tsung N., and Horvitz H. R. (1983). Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics, 104(4), 619–647. 10.1093/genetics/104.4.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treusch S., Hamamichi S., Goodman J. L., Matlack K. E., Chung C. Y., Baru V., Shulman J. M., Parrado A., Bevis B. J., Valastyan J. S., Han H., Lindhagen-Persson M., Reiman E. M., Evans D. A., Bennett D. A., Olofsson A., DeJager P. L., Tanzi R. E., Caldwell K. A., Caldwell G. A., … Lindquist S. (2011). Functional links between Aβ toxicity, endocytic trafficking, and Alzheimer’s disease risk factors in yeast. Science (New York, N.Y.), 334(6060), 1241–1245. 10.1126/science.1213210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski J. Q., Schmidt M. L., Shin R. W., Bramblett G. T., Rao D., and Lee V. M. (1993). Altered tau and neurofilament proteins in neuro-degenerative diseases: diagnostic implications for Alzheimer’s disease and Lewy body dementias. Brain pathology (Zurich, Switzerland), 3(1), 45–54. 10.1111/j.1750-3639.1993.tb00725.x [DOI] [PubMed] [Google Scholar]
- Tsutsui K., Kim H. S., Yoshikata C., Kimura K., Kubota Y., Shibata Y., Tian C., Liu J., and Nishiwaki K. (2021). Repulsive guidance molecule acts in axon branching in Caenorhabditis elegans. Scientific reports, 11(1), 22370. 10.1038/s41598-021-01853-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuovinen T., Kananen J., Rajna Z., Lieslehto J., Korhonen V., Rytty R., Mattila H., Huotari N., Raitamaa L., Helakari H., Elseoud A. A., Krüger J., LeVan P., Tervonen O., Hennig J., Remes A. M., Nedergaard M., and Kiviniemi V. (2020). The variability of functional MRI brain signal increases in Alzheimer’s disease at cardiorespiratory frequencies. Scientific reports, 10(1), 21559. 10.1038/s41598-020-77984-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro A., Tauffenberger A., Aggad D., Rouleau G., Drapeau P., and Parker J. A. (2012). Mutant TDP-43 and FUS cause age-dependent paralysis and neurodegeneration in C. elegans. PloS one, 7(2), e31321. 10.1371/journal.pone.0031321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ham T. J., Thijssen K. L., Breitling R., Hofstra R. M., Plasterk R. H., and Nollen E. A. (2008). C. elegans model identifies genetic modifiers of alpha-synuclein inclusion formation during aging. PLoS genetics, 4(3), e1000027. 10.1371/journal.pgen.1000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Gadea A., Topper S., Young L., Crisp A., Kressin L., Elbel E., Maples T., Brauner M., Erbguth K., Axelrod A., Gottschalk A., Siegel D., and Pierce-Shimomura J. T. (2011). Caenorhabditis elegans selects distinct crawling and swimming gaits via dopamine and serotonin. Proceedings of the National Academy of Sciences of the United States of America, 108(42), 17504–17509. 10.1073/pnas.1108673108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. W., Iturria-Medina Y., Strandberg O. T., Smith R., Levitis E., Evans A. C., Hansson O., Alzheimer’s Disease Neuroimaging Initiative, and Swedish BioFinder Study (2020). Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nature communications, 11(1), 2612. 10.1038/s41467-020-15701-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel K. A., Zhang K., Brodbeck J., Daub A. C., Sharma P., Finkbeiner S., Cui B., and Mucke L. (2010). Tau reduction prevents Abeta-induced defects in axonal transport. Science (New York, N.Y.), 330(6001), 198. 10.1126/science.1194653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., and Chalfie M. (1989). The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes & development, 3(12A), 1823–1833. 10.1101/gad.3.12a.1823 [DOI] [PubMed] [Google Scholar]
- Wegmann S., Maury E. A., Kirk M. J., Saqran L., Roe A., DeVos S. L., Nicholls S., Fan Z., Takeda S., Cagsal-Getkin O., William C. M., Spires-Jones T. L., Pitstick R., Carlson G. A., Pooler A. M., and Hyman B. T. (2015). Removing endogenous tau does not prevent tau propagation yet reduces its neurotoxicity. The EMBO journal, 34(24), 3028–3041. 10.15252/embj.201592748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., and Brenner S. (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 314(1165), 1–340. 10.1098/rstb.1986.0056 [DOI] [PubMed] [Google Scholar]
- Wu Z., Cardona E. A., Cohn J. A., and Pierce J. T. (2025). Nonapoptotic role of EGL-1 in exopher production and neuronal health in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America, 122(2), e2407909122. 10.1073/pnas.2407909122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Ghosh-Roy A., Yanik M. F., Zhang J. Z., Jin Y., and Chisholm A. D. (2007). Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proceedings of the National Academy of Sciences of the United States of America, 104(38), 15132–15137. 10.1073/pnas.0707001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y., Higuchi M., Zhang B., Huang S. M., Iwata N., Saido T. C., Maeda J., Suhara T., Trojanowski J. Q., and Lee V. M. (2007). Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron, 53(3), 337–351. 10.1016/j.neuron.2007.01.010 [DOI] [PubMed] [Google Scholar]
- Zhou W., Scott S. A., Shelton S. B., and Crutcher K. A. (2006). Cathepsin D-mediated proteolysis of apolipoprotein E: possible role in Alzheimer’s disease. Neuroscience, 143(3), 689–701. 10.1016/j.neuroscience.2006.08.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data can be found either within the article or in its supplementary information section. Raw data is available upon request.