Summary
Here, we present a protocol that models peripheral artery disease using the surgical induction of hindlimb ischemia (HLI) in mice. We list the steps to surgically excise the femoral artery and assess blood flow recovery through laser Doppler imaging. We then detail procedures for visualizing immune cell recruitment and blood vessel growth to explore the role of inflammatory macrophages in angio/arteriogenesis.
For complete details on the use and execution of this protocol, please refer to Mantsounga et al.1
Subject areas: Health Sciences, Immunology, Microscopy, Model Organisms, Molecular Biology
Graphical abstract

Highlights
-
•
Surgically induce hindlimb ischemia using an experimental model of PAD
-
•
Assess and quantify blood flow recovery using laser Doppler imaging
-
•
Harvest tissue to isolate DNA, RNA, and protein for further analysis
-
•
Quantify immune cell recruitment and blood vessel growth through immunofluorescence
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Here, we present a protocol that models peripheral artery disease using the surgical induction of hindlimb ischemia (HLI) in mice. We list the steps to surgically excise the femoral artery and assess blood flow recovery through laser Doppler imaging. We then detail procedures for visualizing immune cell recruitment and blood vessel growth to explore the role of inflammatory macrophages in angio/arteriogenesis.
Before you begin
The femoral artery ligation model of peripheral artery disease allows quantitation of angiogenesis and arteriogenesis in response to vascular injury. This model is particularly useful in studying mechanisms of immune and vascular response to induced ischemia.
The protocol below sequentially describes procedures for
-
1.
the surgical excision of the femoral artery (induction of HLI)
-
2.
laser Doppler imaging to assess blood flow recovery
-
3.
immunofluorescence histology for assessment of immune and endothelial cell recruitment and new blood vessel growth
Note: The mouse lines described in this protocol are related to the original manuscript by Mantsounga et al.1 This protocol details key steps used in that manuscript1 to execute the experimental model of PAD (HLI) conducted to assess changes to blood flow recovery between control (C57BL/6J) and myeloid IL-1β-deleted mice. This protocol can be adapted to other experimental designs using other mouse lines of interest. Questions regarding the experimental rationale and subsequent discussion should be referred to the original manuscript.
Institutional permissions
All experiments were performed in accordance with the Providence VA Medical Center guidelines, and the Institutional Animal Care and Use Committee approved all housing protocols and experimental animal procedures. All data, analytical methods, and study materials are available upon request. Personal protective equipment, autoclaved sterile surgical instruments, and aseptic technique are essential for handling small animals. Please ensure that permissions are granted by your institution through the appropriate regulatory agencies (IACUC, Research and Safety Committees, IBC, etc.) prior to engaging in any animal work detailed within this protocol.
Surgical excision of the femoral artery (HLI)
Preparing surgical tools
Timing: 30 min
This section describes how to properly prepare surgical tools to prevent infection in the animals from surgery.
-
1.Autoclave a set of surgical instruments in an autoclavable case within an autoclave sterilization pouch (Figure 1)
-
a.1 Dumont forceps #5/45
-
b.1 Dumont forceps micro-blunted tips
-
c.1 curved serrated forceps or Adson’s forceps
-
d.1 Vannas scissors
-
e.1 dissecting scissors
-
f.1 needle holder
-
g.9 mm wound closure clips
-
h.Wound closure clamp
-
a.
-
2.
Autoclave surgical gauze within a sterilization pouch
-
3.
Autoclave ∼6 × 10 inch sections of surgical drapes within a sterilization pouch
-
4.
Autoclave ∼5 inch squares of aluminum foil within a sterilization pouch
Figure 1.
Set of surgical instruments in an autoclavable case
Preparing animal cages
Timing: 5 min
This section describes preparing fresh animal cages that the animals will be put in after surgery to recover in a clean, warm environment.
-
5.
Prepare fresh housing cages equal to the number of animals undergoing surgery, filling the empty cages with enrichment, bedding, food, and water
-
6.
Place a recirculating heating pad under the fresh cage so that half of the cage is on and half of the cage is off of the heating pad
-
7.
Create surgical documentation cards (often required by your institution) for detailing surgery events and post-operative monitoring
Preparing anesthesia
Timing: 5 min
This section describes preparing the anesthesia machines that will be used on all mice undergoing surgery.
-
8.Prepare two anesthesia stations (Figure 2)
-
a.Place an anesthesia machine connected to a mouse chamber and connected to a tube with a nose cone onto one side of the counter. This will become the animal aseptic preparation station
-
b.Place a second anesthesia machine and second tube with a nose cone at the end onto the other side of the counter. This will become the sterile surgical station
-
a.
-
9.Ensure both anesthesia machines and chambers are adequately supplied
-
a.Fill isoflurane to the appropriate level
-
b.Check that the oxygen tank is full and completely opened
-
c.Weigh the charcoal scavenger containers and replace the container once the weight is approaching the manufacturer’s specified limit
-
a.
Note: Sufficient levels of 1–3% isoflurane with O2 must be flowing through the chamber/nose cone to ensure proper anesthesia of the mouse during the entirety of the surgical procedure
-
10.
Lay a fresh section of paper towel onto the floor of each anesthesia chamber
Note: Paper towel should be changed between subsequent mice
Figure 2.
Anesthesia station with mouse chamber, oxygen tank, and isoflurane vaporizer
Preparing the surgical space
Timing: 15 min
This section describes properly preparing a sterile area for surgery to prevent infection in the animals from surgery.
-
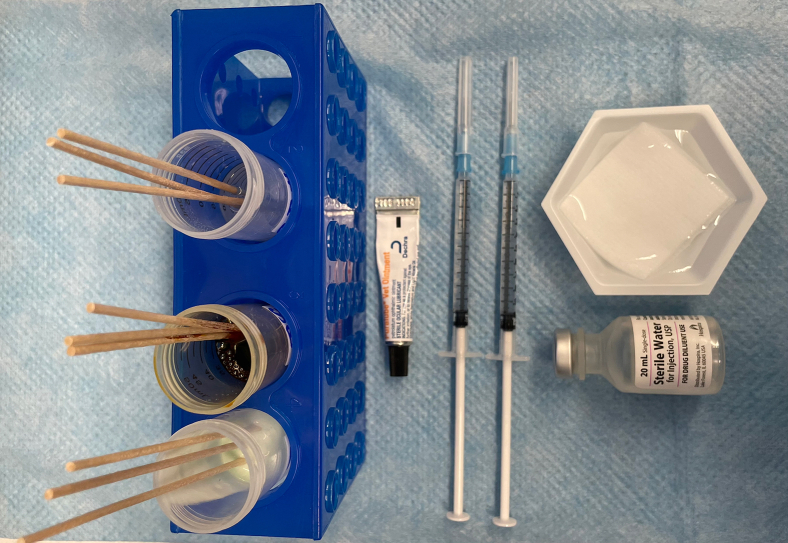
11.Prepare aseptic preparation station by placing surgical tape, a sterile 50 mL centrifuge tube filled with Nair, two 1 mL syringes filled with sterile water, a sterile 50 mL centrifuge tube filled with betadine, a sterile 50 mL centrifuge tube filled with 70% isopropyl alcohol, and a tube of petrolatum ophthalmic ointment onto a sterile absorbent pad for this station, filling each Falcon tube to at least 20 mL of respective reagent (Figure 3)
-
a.Place three sterile swabs in each 50 mL falcon tube per animal (Figure 4)
-
b.Affix the anesthesia nose cone on top of the sterile absorbent pad with surgical tape
-
a.
-
12.Prepare sterile surgical station
-
a.Place dissecting microscope on the surgical counter space (Figure 5)
-
b.Place a recirculating heating pad followed by a sterile absorbent pad over the base of the microscope (Figure 6)
-
c.Affix the anesthesia nose cone on top of the sterile pad with surgical tape, ensuring that the nose cone rests within range of the microscope’s lens (Figure 7)
-
d.Open the sterilization bags/packaging to expose the sterile gloves, autoclaved tools, autoclaved gauze, autoclaved drapes, sterile absorbent pad, and sterile sutures, but do not directly touch or allow contact of any of the equipment to any surfaces outside of the sterilization bags/packaging at this time. Tools should be next to the microscope to be used during surgery (Figure 8)
-
e.Lightly place two pieces of surgical tape upon the microscope absorbent pad to be; used later for stabilizing the mouse’s body
-
f.Prepare pain medication to appropriate dosage to be used immediately after surgery as set forth in the Guide for the Care and Use of Laboratory Animals.2 In this case, the specific dosage is noted below.Note: There are protocols that describe using other pain medications. This protocol uses buprenorphine at a dosage of 0.05–0.1 mg/kg, SC BID x 3 days.
-
g.Set aside corresponding number of sterile syringes to mice onto sterile pad with other equipment
-
a.
Figure 3.
Materials for aseptic preparation station: Nair lotion, sterile betadine solution, 70% isopropyl alcohol, petrolatum ophthalmic ointment, gauze soaked in water, sterile water, and two syringes filled with 1 mL of sterile water
Figure 4.
3 swabs placed into each 50 mL falcon tube per animal for the aseptic preparation station
Figure 5.
Dissecting microscope on surgical counter space
Figure 6.
Dissecting microscope with recirculating heating pad and absorbent pad
Figure 7.
Anesthesia cone placement upon dissecting microscope
Figure 8.
Sterile surgical tools within bag
(A) Bags placed in optimal position.
(B) Bags opened to maintain sterility.
Laser Doppler imaging
Preparing Doppler equipment
Timing: 10 min
This section describes setting up the laser Doppler equipment before anesthetizing the animals.
-
13.Turn on a recirculating heating pad under the Doppler machine
-
a.If the heating pad is not black, cover the heating pad with a black pad or drape to prevent light reflection that may interfere with the laser Doppler equipment
-
a.
-
14.
Set up a heating lamp to be aimed at the heating pad under the Doppler machine but 1–2 feet away (Figure 9)
-
15.
Connect anesthesia as detailed in steps 8a and 9 so that the animal will receive continuous flow of anesthesia while on the heating pad during the duration of the laser Doppler session
-
16.Turn on the rectal probe for body temperature
-
a.Fill a 50 mL tube with sterile water that will be used to cleanse the probe before and after each mouse
-
b.Cleanse the probe by dipping the probe into the water, swirling the probe, and wiping away the excess water with Kimwipes
-
a.
-
17.Open MoorLDI software for laser Doppler imaging (Figure 10)
-
a.Use the software prompts to auto-select a scanning box that encompasses the hind paws in entirety (see step 41 below for paw positioning)
-
a.
Figure 9.
Laser Doppler machine with heating lamp, anesthesia nose cone, and supporting software
Figure 10.
Laser Doppler software setup box
Immunofluorescence staining
Preparing reagents
Timing: 30 min
This section describes preparing reagents for staining that can be made ahead of time
-
18.
Prepare PBS (1X) Solution
-
19.Prepare 50 mL of PBLec Solution
-
a.PBS with 1% Triton X-100, 1 mM CaCl2, 1 mM MgCl2, 0.1 mM MnCl2
-
a.
-
20.
Aliquot 1.5 mL of Goat Serum to be used when preparing blocking buffer with a working concentration of 5% (v/v) Goat Serum
-
21.Prepare antibodies of interest
-
a.Centrifuge antibodies at 200 g for 10 min at 4 prior to use
-
b.Reconstitute antibodies as directed
-
c.Dilute the antibodies to the appropriate concentration using blocking buffer (see below for concentrations used in this protocol)
-
a.
Note: In this protocol, we use DAPI in mounting media, CD31-FITC (0.5 mg/mL), CD68-APC (0.2 mg/mL), and SMA-Cy3 (1.0–1.5 mg/mL)
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| ProLong gold antifade mountant with DAPI | Thermo Fisher Scientific | Cat# P36931 |
| CD31 (PECAM-1) monoclonal antibody (390), FITC, eBioscience (stock: 0.5 mg/mL; final: 5 μg/mL) | Thermo Fisher Scientific | Cat# 11-0311-85 |
| APC anti-mouse CD68 antibody (stock: 0.2 mg/mL; final 2 μg/mL) | BioLegend | Cat# 137008 |
| Anti-actin, α-smooth muscle-Cy3 antibody, mouse monoclonal (stock: 1.0–1.5 mg/mL; final 10–15 μg/mL) | MilliporeSigma | Cat# C6198 |
| Chemicals, peptides, and recombinant proteins | ||
| Buprenorphine, 0.05–0.1 mg/kg | Par Pharmaceutical | Cat# NDC42023-179-01 |
| PBS, phosphate-buffered saline, 10X solution, Fisher BioReagents | Fisher Scientific | Cat# BP3991 |
| Triton X-100 | Santa Cruz Biotechnology | CAS# 9002-93-1 |
| Calcium chloride solution (CaCl2) (stock: 1.0 M) | Honeywell Fluka | Cat# 21114-1L |
| Magnesium chloride (MgCl2) (stock: 25 mM) | Thermo Scientific | Cat# R0971 |
| Manganese(II) chloride (MnCl2), 50 g | Sigma-Aldrich | Cat# 244589-50G |
| Goat serum | MilliporeSigma | Cat# G9023-10ML |
| 0.9% sodium chloride for injection, USP | Hospira, Inc. | NDC 0409-4888-02 |
| Sterile water for injection, USP | Hospira, Inc. | NDC 0409-4887-23 |
| Invitrogen TRIzol LS reagent | Fisher Scientific | Cat# 10-296-010 |
| Paraformaldehyde solution, 4% in PBS, Thermo Scientific Chemicals | Thermo Fisher Scientific | Cat# J19943.K2 |
| Sucrose (EP/BP/NF), Fisher Chemical | Fisher Scientific | Cat# S2-500GM |
| O.C.T. compound | Fisher Scientific | Cat# 23-730-571 |
| Experimental models: Organisms/strains | ||
| Mus musculus: C57BL/6 mice (10- to 12-week-old males and females) | The Jackson Laboratory | Cat# 000664; RRID: IMSR_JAX:000664 |
| Mus musculus: Csf1rmercremer, FVB-Tg(Csf1rcre/Esr1∗)1Jwp/J (10- to 12-week-old males and females) | The Jackson Laboratory | Cat# 019098; RRID: IMSR_JAX:019098 |
| Mus musculus: IL-1 fl/fl (10- to 12-week-old males and females) | The Morrison Laboratory and the Brown University Mouse Transgenic and Gene Targeting Facility | Mantsounga et al.1 |
|
Mus musculus: Ai9 (RCL-tdT), B6.Cg-Gt(ROSA) 26Sortm9(CAG-tdTomato)Hze/J (10- to 12-week old males and females) |
The Jackson Laboratory | Cat# 007909; RRID: IMSR_JAX:007909 |
| Software and algorithms | ||
| moorLDI version 6.1 | Moor Instruments | https://www.moor.co.uk/en-us/products/control/moorldi2-research-software/ |
| ImageJ version: 2.0.0-rc-69/1.52p build:269a0ad53f date 2018-12-04T11:30:09 + 000 | Schneider et al., 2012 | https://imagej.net/software/fiji/downloads |
| Other | ||
| Instrument sterilization tray with silicone mat, 15 × 25.5 × 2 cm | World Precision Instruments | Cat# 500254 |
| Crosstex international pouch sterilization duo check, 12 × 18 in | Grayline Medical | Cat# SCL1218 |
| Dumont #5/45 forceps, standard/Dumoxel | Fine Science Tools | Cat# 11251-35 |
| Dumont forceps micro-blunted tips | Fine Science Tools | Cat# 11253-20 |
| Curved serrated forceps/Adson’s forceps from mouse surgical kit | Kent Scientific | Cat# INSMOUSEKIT |
| Vannas scissors, 8 cm, 5 mm blades, straight | World Precision Instruments | Cat# 14003 |
| Dissecting scissors, 10 cm, straight | World Precision Instruments | Cat# 14393 |
| Webster needle holder, serrated | World Precision Instruments | Cat# 14109 |
| AutoClip 9 mm wound closure clip | ALZET | Cat# 0009953 |
| Wound closure clamp | World Precision Instruments | Cat# 503292 |
| Covidien 9132 Curity all-purpose sponges, non-woven, 3-ply, 2″ × 2″ size | Vitality Medical | Cat# 9132 |
| Medium sheet, 40 × 71 in | Cardinal Health | Cat# 9355 |
| BWK7110, Boardwalk premium quality aluminum foil roll | Amazon | Cat# B00H615JA2 |
| Far infrared warming pad with controller | Kent Scientific | Cat# RT-0515 |
| Midmark VMR non-rebreathing anesthesia machine | Midmark | Cat# 91800218 |
| Sterile absorbent pad | HK Surgical | Cat# PD-X |
| 3M Transpore surgical tape, 1 in (width) | Fisher Scientific | Cat# 18-999-381 |
| 50 mL centrifuge tube, bag, sterile | Fisher Scientific | Cat# 50-202-003 |
| Nair hair remover lotion with soothing aloe & lanolin | Medline | Cat# C-H22329 |
| BD 1 mL TB syringe | Vitality Medical | Cat# 309626 |
| Povidone iodine prep solutions | Medline | Cat# MDS093906H |
| Decon CiDehol 70 isopropyl alcohol solution | Fisher Scientific | Cat# 04-355-71 |
| Puralube veterinarian ophthalmic ointment | Covetrus | Cat # 008897 |
| Sterile cotton swabs | Medline | Cat# MDS202095Z |
| Leica stereo microscope | Leica Biosystems | Model M80 |
| Sterile gloves | Thomas Scientific | Cat# 20A00C896, 20A00C 897, 20A00C898 |
| 7-0 DemeTECH DemeLENE polypropylene blue 18″ P-1 reverse cutting sutures | eSutures.com | Cat# PM197011F13M |
| High-resolution laser Doppler imager | Moor Instruments Ltd. | Cat# MOORLDI2-HIR |
| Heating pad | Harvard Apparatus | Cat# 55-7020 |
| Enterprise Technology Solutions latex-free mouse pad, 9 × 7.5, black | Fisher Scientific | Cat# 50-233-4833 |
| Heat lamp | Braintree Scientific | Cat# HL-1 120V |
| Kimberly-Clark Professional Kimtech Science Kimwipes delicate task wipers 1-ply | Fisher Scientific | Cat# 06-666 |
| Inotech Biosceince LLC IS-350 dry sterilizer | Fisher Scientific | Cat# NC9449759 |
| Flat razor blade | VWR | Cat# 55411-050 |
| Tissue-Tek Cryomold disposable vinyl specimen molds 25 × 20 × 5 mm | VWR | Cat# 25608-916 |
| Leica CM3050 S cryostat | Leica Biosystems | Model CM3050 S |
| Simport Scientific StainTray slide staining systems, black lid, only for M922 stain tray, M923-2 | Grayline Medical | Cat #M923-2 |
| ImmEdge pen | Vector Labs | Cat# H-4000 |
| Nikon ECLIPSE Ni-E microscope | Nikon | Model Ni-E |
Materials and equipment
PBlec
| Reagent | Concentration | Amount |
|---|---|---|
| PBS | 1X | 94.9 mL |
| Triton X-100 | 1.6 mM (1% v/v) | 1 mL |
| CaCl2 | 1 mM | 100 μL |
| MgCl2 | 1 mM | 4 mL |
| MnCl2 | 0.1 mM | 1.2584 g |
| Total | 1X | 100 mL |
Store at 4 for up to three months
PBS-Sucrose
| Reagent | Concentration | Amount |
|---|---|---|
| PBS | 1X | 100 mL |
| Sucrose | N/A | 30 g |
| Total | 1X | 100 mL |
Store at 4 for up to three months
Step-by-step method details
Femoral artery ligation
Anesthetize and prepare mouse for surgery
Timing: 5 min per mouse
This section describes the proper preparation of the mouse going into surgery and is critical for reducing postoperative infections and complications that may affect the survival outcomes of the mice.
-
1.
Obtain pre-surgical weights and record this weight
Note: Weights should be recorded for all animals undergoing these procedures to ensure that they do not lose more than 10–15% of their weight. Animals that have lost >15% of their body weight or appear lethargic or moribund will be euthanized.
-
2.Place mouse into anesthesia chamber lined with paper towel with only O2 flowing.
-
a.Secure the chamber lid, and allow 1–3% isoflurane to enter the chamber
-
a.
-
3.
Monitor mouse in chamber
Note: The percentage of isoflurane should be adjusted to ensure that the mouse enters a surgical plane of anesthesia slowly
Note: Mouse is fully anesthetized when it has no reaction to toe pinch
-
4.
Transfer mouse from the anesthesia chamber to the nose cone affixed onto the aseptic preparation station oriented in the sternal recumbent position with the ventral side touching the sterile pad (Figure 11)
CRITICAL: Mouse should be kept warm by a recirculating heating pad if under anesthesia for more than 5 min.
-
5.
Immediately apply sterile ophthalmic ointment (such as PuraLube ointment—see Key Resources) to both eyes
CRITICAL: Ophthalmic ointment is critical for prevention of postoperative blindness from desiccation, indicative to certain strains of mice
-
6.Gently turn mouse over to the dorsal recumbent position so that the dorsal side of the mouse rests on the absorbent pad
-
a.Secure the mouse’s hindlimbs using the pre-sectioned pieces of tape (Figure 12)Note: Hindlimbs should be abducted to the sides so that the skin is taut throughout the limb at the level of the inguinal ligament
-
a.
-
7.Apply Nair using a sterile swab to the limb undergoing surgery, removing an area of hair that is 50% greater than the length of the incision (see Methods video S1)
-
a.Allow the Nair to soak for roughly 45 s, wipe with moistened gauze, and gently wash any remaining Nair with sterile water
-
b.If needed, repeat Nair application with a fresh swab to completely remove hair from the area
-
c.Repeat wash steps with sterile water to cleanse off the area until all the Nair lotion is removed (Figures 13 and 14)Note: Any residual Nair lotion remaining on the skin may cause chemical burns to the animal and should be avoidedNote: Either hindlimb may be used in this procedure, however, the same limb should be used between animals to ensure consistency
-
a.
-
8.Apply betadine followed by 70% isopropyl alcohol using pre-soaked sterile cotton swabs moving in concentric circles starting from the center and rotating outward over the prepped area (See Methods video S2)
-
a.Repeat this step two more times (3 times total) using a new pre-soaked sterile cotton swab each time
-
a.
Note: At this point, the animal is aseptically prepped and ready to be transferred to the surgical area
Figure 11.
Representation of mouse in sternal recumbent position for ophthalmic ointment application prior to surgery
Figure 12.
Representation of mouse in dorsal recumbent position with hind paws taped for stability during surgery
Figure 13.
Representation of mouse in dorsal recumbent position with hair removed from the surgical area
Figure 14.
Mouse in dorsal recumbent position with hair removed from surgical area
-
9.
Turn on the surgical anesthesia station, ensuring that 1–3% isoflurane with O2 is flowing through the nose cone
-
10.
Transfer the mouse to the sterile surgical station so that the dorsal side of the mouse rests upon the sterile absorbent pad, with the recirculating heating pad below the sterile absorbent pad (Figure 15)
-
11.
Insert the rectal probe for body temperature monitoring
-
12.
Remove gloves and put on sterile gloves
Note: Surgical glove technique should ensure no contamination of the gloves by skin or equipment contact
-
13.
Affix autoclaved aluminum foil to the knobs of the microscope to adjust the focus throughout the procedure to maintain sterility
-
14.
Cut an opening on the autoclaved drapes for surgical draping using the autoclaved dissecting scissors
-
15.
Apply the drape onto the mouse, leaving the head and nose cone exposed for continuous monitoring throughout the procedure and the pre-sectioned hole over the prepped limb undergoing surgery
Figure 15.
Representation of aseptically prepared mouse in dorsal recumbent position, placed onto dissecting microscope station for surgery
Perform a two-stage scrub on the hindlimb with betadine followed by 70% isopropyl alcohol.
Ligation and excision of the femoral artery
Timing: 10 min per mouse
This section describes the ligation and excision of the femoral artery that will induce ischemia in the limb. The femoral artery is the major vessel supplying blood flow to the adductors of the thigh, gastrocnemius, soleus, and portions of the hamstring muscles. Loss of this arterial blood supply stimulates the growth of new blood vessels, both capillaries and arterioles as well as pre-existing collateral networks. Depending on the genetic modifications of the mice used, there may be differences in the ability to grow new blood vessels, resulting in altered kinetics of blood flow recovery as measured by laser Doppler imaging.1
-
16.Using blunt serrated forceps, gently pinch the skin at the level of the inguinal ligament
-
a.Make a 45-degree incision with dissecting scissors that extends 5 mm (Figures 16 and 17) (see Methods video S3)
-
a.
-
17.
Using Dumont forceps, gently clean away the superficial white adipose tissue until the femoral artery, femoral vein, and femoral nerve are exposed (see Methods video S4)
Note: The femoral artery will appear as a thin, pale pink band above a dark red vein, with the head of the vein appearing bulbous and large
Note: If femoral artery cannot be identified, you may have made the incision in the incorrect location, and you should refer to Troubleshooting Problem 1
Note: When exposing the femoral artery, femoral vein, and femoral nerve, be careful to pull apart the fat tissue by gently pulling in opposing directions. Some mice may have less fat tissue than others; it is important to recognize this superficial tissue needs to only be separated apart to expose the area. Removal of fat tissue is not necessary, but may help in visualization, especially in obese mice
Note: Superficial vessels may be ruptured while pulling apart fat tissue. If this is the case, then pressure should be applied using a sterile swab until bleeding stops before the procedure is removed.
Note: If there is significant bleeding, you may have perforated the femoral artery or vein, and you should refer to Troubleshooting Problem 2
-
18.
Using a curved Dumont forceps, first isolate the nerve away from the femoral artery and femoral vein by flexing the forceps (Figure 18) (see Methods video S5)
-
19.Carefully isolate the femoral artery from the femoral vein, beginning on the proximal end, then use 7-0 polypropylene sutures to ligate the femoral artery at two positions, spaced 5 mm apart: one just below the inguinal ligament (proximal) and the second at the intersection of the superficial epigastric artery and femoral artery (distal) (Figure 19)
-
a.While holding the end of a suture with straight Dumont forceps, use other hand to feed the curved #5/45 Dumont forceps under the femoral artery, gently flexing the forceps open
-
b.Carefully transfer the suture thread to the curved Dumont forceps and pull the suture thread in a continuous motion back under the femoral artery, adjusting the suture thread to either the proximal or distal end of the femoral artery (see Methods video S6)Note: It may be easier to first ligate the proximal end of the femoral artery followed by the distal end of the femoral artery to ensure sufficient space between sutures for excision
-
c.Ligate the artery using an instrument tie (see Methods video S7)
-
d.Use dissecting scissors to trim the ends of the suture as closely as possible (see Methods video S8)
-
e.Repeat a-d on the other side of femoral artery to secure the femoral artery in two positions (see Methods videos S9 and S10)Note: These vessels can be easily injured if care is not taken in the dissection process. It is of utmost importance to ensure that there is no perforation of the femoral artery prior to ligation of both ends
-
a.
-
20.
Once both ends are secured, flex the Dumont forceps to fully separate the femoral artery from the femoral vein (see Methods video S11)
-
21.
Use Vannas scissors to excise the 5 mm segment of femoral artery between the knots. There should be no bleeding. Visually confirm the excision of the artery (see Methods video S12)
-
22.
To close the surgical wound, bring both ends of the skin together with the blunt serrated forceps and secure the wound with a 9 mm wound clip, firmly closing it with a wound clip clamp (see Methods video S13)
-
23.
With a sterile cotton swab, wipe the surgical area with betadine (see Methods video S14)
-
24.
Remove the tape from the hindlimbs
-
25.
Inject up to 1 mL of warm sterile saline SC at this time to prevent dehydration
-
26.Place the mouse into the clean housing cage which is positioned half on and half off a water based recirculating heating pad
-
a.Monitor the mouse until it is fully awake, alert, and ambulatory
-
a.
Figure 16.
Representation of mouse in dorsal recumbent position ready for surgery with marked incision site
Figure 17.
Image of mouse in dorsal recumbent position with incision site marked
Figure 18.
Surgical area with labeled anatomy
Figure 19.
Surgical area with labeled anatomy
Yellow hash marks indicate site of sutures.
Transitioning to the next mouse
Timing: 15 min
This step will allow for sterilization between mice to reduce risk of postoperative infection. This should be done before placing the next mouse into the anesthesia chamber.
-
27.
Using fresh sterile gloves, wipe all tools clean of any remaining tissue debris or blood using sterile surgical gauze and sterile water
-
28.
Sterilize the tips of the tools in a bead sterilizer before returning them to the tool kit
Note: The tips only method of bead sterilization is appropriate for up to 5 animals, after which the tools must undergo autoclave sterilization
Note: Alternatively, a freshly autoclaved set of tools may be used for each mouse, in which case steps 27 and 28 can be skipped
-
29.
Replace both absorbent pads in the preparation and the surgical area
-
30.
Prepare the next mouse for surgery by going back to step 1
Post-procedural care and monitoring
Timing: 3–21 days
This step ensures mice are cared for and doing well after surgery.
-
31.After surgery, allow animals to recover in a warm and dry area of the cage with access to food and water until they are awake, alert, ambulatory, and able to thermoregulate. This is evidenced by the ability to move around normally within the cage
-
a.Document all operative and immediate postoperative details in postoperative care forms
-
a.
-
32.Monitor mice and provide buprenorphine analgesic at the given dosage approved by your institution
-
a.Document all analgesic dosage times and assessment of incision appearance on the surgery cards and postoperative care forms
-
a.
-
33.Monitor daily thereafter until euthanized, providing additional doses of buprenorphine as approved by the veterinarian to animals that demonstrate pain or distress beyond the first 3 postoperative days
-
a.Evaluate mice for weight and general health, as well as necrosis of distal tissue. An example of the scoring system for distal necrosis is as follows: 0 (HEALTHY), 1 (BLACK NAIL), 2 (BLACK TOE), 3 (TOE LOSS), 4 (FOOT LOSS). Animals are treated with multimodal analgesia (SCORE 1, 2, OR 3) or euthanized (SCORE 4) (Figure 20)
-
a.
Figure 20.
Necrosis scoring scale
Laser Doppler imaging
Anesthetize and prepare mouse for imaging
Timing: 25 min per mouse
This step is a critical preparation step. Proper preparation reduces postoperative infections and complications that may affect the survival outcomes of the mice.
-
34.
Record the weight of the mouse prior to beginning the protocol
-
35.Place mouse into anesthesia chamber lined with paper towel with only O2 flowing
-
a.Secure the chamber lid, and allow 1–3% isoflurane to enter the chamber
-
a.
-
36.
Monitor mouse in chamber
Note: The percentage of isoflurane should be adjusted to ensure that the mouse enters a surgical plane of anesthesia slowly
Note: Mouse is fully anesthetized when it has no reaction to toe pinch
-
37.
Transfer mouse from the anesthesia chamber to the nose cone affixed onto the heating pad oriented with the ventral side touching the pad
-
38.
Immediately apply sterile ophthalmic ointment to both eyes
-
39.
Confirm that heating pad and heating lamp are on and appropriately spaced
Note: Heat lamp should be at least 1–2 feet away from the mouse.
-
40.
Insert rectal probe to begin measuring core body temperature
Note: Mouse should be at 37 ± 0.5°C at the time of the Doppler scan
Note: It takes about 20 min for the average mouse to reach this temperature
CRITICAL: The mice must reach the described temperature for accurate blood flow recovery assessment
-
41.
Mark the area to be scanned using the moorLDI software. The hind paws of the mouse should be flattened and facing palm side up (Figure 21)
Figure 21.
Laser Doppler imaging software setup with mouse in sternal recumbency placed into margins of the scanning box
Scanning the hindlimbs
Timing: 5 min per mouse
This section describes using the laser Doppler machine to scan the mouse for blood flow visualization. This should be done before surgery to get a baseline reading, right after surgery (day 0), and on postoperative days 3, 7, 14, and 21.
-
42.
Turn off the heat lamp just before beginning the scan. Refer to Troubleshooting Problem 3 for more information.
Note: The heat lamp must be turned off just prior to scanning the paws, as external lights can interfere with scanning results. Ensure that the lamp is switched off during scanning of each animal and that the lamp is kept at the same position between animals to maintain consistency.
-
43.
Begin the scan, continually monitoring to ensure that the paws are flat and that the paws, hindlimbs, and tails are in view throughout the entirety of the scan (Figure 22)
Note: If the paws do not appear flat, and the tail vein is not visible in the scan, you may need to abort the scan and re-adjust before beginning the scan again. Refer to Troubleshooting Problem 4
-
44.
Save the scan for quantification of blood flow recovery over time
-
45.
Transfer mouse back to its cage and allow the mouse to rest in the cage with heating lamp
-
46.Once the animals have fully recovered from anesthesia, as evidenced by sternal recumbency and physical mobility, weigh the mouse again and record the measurement
-
a.Return the cage to the housing room in the animal care facility
-
a.
-
47.
Repeat steps 34–46 on post-surgical day 3, 7, 14, and 21 (see Figure 23 for example of blood flow recovery image outcomes)
Figure 22.
Laser Doppler imaging scan in progress
Paw orientation demonstrated in the color image
Figure 23.
Laser Doppler images of blood flow in the ischemic and contralateral control hindlimbs of control or myeloid IL-1 -deleted mice (mIL-1 KO) at indicated time points before and after femoral artery ligation
Immunofluorescence staining
Harvesting calf muscle tissue
Timing: 15 min
This section describes dissecting the ischemic calf muscle in order to later assess changes at the molecular level within the muscle using techniques such as immunofluorescence, western blot, qPCR. Depending on the experimental design, the contralateral control limb can be dissected as well. Muscle dissection can take place on postoperative day 3 to assess the acute response to injury, as well as on postoperative day 21 to assess the long-term response to injury.
-
48.
Euthanize mice according to your institution’s guidelines to ensure humane and proper euthanasia.
-
49.
Place euthanized mouse upon a fresh absorbent pad
Note: Clean tools, fresh gloves, and a fresh absorbent pad should always be used for optimal results
-
50.
Carefully remove the skin of the hindlimb to expose the underlying musculature (Figure 24)
-
51.
Using dissecting scissors, insert the tip of the closed scissors under the gastrocnemius tendon, flexing it open to isolate the gastrocnemius and soleus muscle from the bone (Figure 25)
Note: Although the gastrocnemius is the major muscle tissue isolated and often referenced, the soleus muscle is closely connected to the gastrocnemius muscle and is often included in this isolation.
-
52.
Holding the tendon with forceps in one hand, carefully dissect up the muscle to an additional 3–5 mm of biceps femoris
-
53.Lay the muscle flat, cut the muscle in one motion using a flat blade to set aside 3–5 mm of gastrocnemius that should still be connected to the 3–5 mm of biceps femoris (Figure 26)
-
a.Set aside this piece into TRIzol for further quantitative DNA, RNA, or protein analysis
-
a.
Note: Although not described in this protocol, TRIzol reagent is particularly useful in isolating DNA, RNA, and protein from a large sample, such as animal tissue. These samples are useful in analyzing the quantitative changes of gene or protein expression through techniques such as qPCR or Western blot. To further explore the application and interpretation of these techniques, please refer to our previous manuscript.1
-
54.
Place the remaining intact gastrocnemius tendon and gastrocnemius muscle into 4% PFA for histological analysis
Figure 24.
Musculature exposed with arrow indicating gastrocnemius muscle
Figure 25.
Dissecting scissors under gastrocnemius muscle to isolate from underlying bone
Figure 26.
Cutting the gastrocnemius muscle tissue
(A) Isolated gastrocnemius muscle tissue with tendon.
(B) Forceps gripping the tendon while the blade cuts through the muscle.
Tissue fixation
Timing: 5 days
This section describes preparing the muscle tissue for cryosectioning.
-
55.
Store gastrocnemius muscle in 4% PFA at 4°C for 12–24 h
-
56.
Transfer the muscle to PBS-Sucrose for an additional 48–72 h, until the tissue sinks to the bottom of the falcon tube
-
57.
Arrange an enclosed Styrofoam container filled with dry ice onto the lab bench
-
58.Prepare the muscle for cryosectioning by placing the tissue into a cryomold filled with OCT, ensuring that the cut edge of the muscle lies on the bottom of the mold, with the tendon standing straight towards the top to ensure proper plane of cross-sectioning of the muscle tissue
-
a.Place cryomold upon dry ice with a closed lid for 3–5 min and wait until the mold is opaque. This color change of the OCT from clear to opaque indicates that the sample is frozen and ready for storage (Figure 27)
-
b.Transfer molds to −80°C for storage
-
a.
Note: PBS-Sucrose functions as a cryoprotectant. Ensure that OCT is placed smoothly with no bubbles. Freezing of the mold using dry ice should done quickly and efficiently to prevent any crystallization or cracks in the tissue.
Figure 27.
Cryomolds with tissue showing process of freezing
(A) Tissue placed into OCT compound.
(B) Cryomold placed upon dry ice.
(C) Fully frozen sample ready for storage.
Cutting frozen sections
Timing: 10 min
This section describes creating cut sections of frozen muscle tissue to be placed on microscope slides.
-
59.
Cut 8-10-micron thick frozen sections of the calf muscle using a cryostat machine
-
60.
Each slide should have two 8-10-micron sections
-
61.
Save the block in −80°C for future sectioning purposes
Note: While the likelihood of tissue degradation is lower due to fixation protocol prior to embedding, is it good practice to stain sections with hematoxylin & eosin to ensure preservation of tissue architecture (Figure 28).
Figure 28.
Representative images of immunohistochemistry for F4/80 on ischemic muscle gastrocnemius muscle tissue from day 3 post femoral artery ligation, demonstrating macrophage infiltration and preservation of muscle tissue architecture
Bar, 50 μm.
Preparing slides
Timing: 40 min
This section describes preparing the frozen sections of muscle tissue for staining.
-
62.Prepare a humid staining tray using wet paper towel
-
a.Soak two sheets of paper towel in water and place them flat onto the dark staining tray (Figure 29)
-
a.
Note: Using moist paper towel ensures that the tray will remain humid without any liquid spilling onto slides during any transferring or physical movement of the tray
-
63.Place each slide with a frozen cryosection onto the tray
-
a.Let the slides come to room temperature (25°C) (about 15 min), circle the section with a hydrophobic pen, and wait an additional 10 min for the hydrophobic barrier to dry
-
a.
-
64.
Wash the slides in PBS 1X, cover with the tray lid, and allow to sit for 15 min at room temperature
Figure 29.
Moistened paper towel placed onto dark staining tray
Blocking step
Timing: 1 h
This section describes adding blocking buffer to the microscope slides before antibody staining to block the antibodies from binding to nonspecific targets.
-
65.
Make a blocking buffer comprised of 5% (v/v) Goat Serum in cold PBlec 1X
-
66.
Pipet about 300 μL of blocking buffer onto each slide, cover, and allow to block for 1 h at room temperature (25°C)
Primary antibody incubation
Timing: 10 min, then overnight incubation (∼12–16 h)
This section describes staining with primary antibodies of interest to the microscope slides.
-
67.
Dilute primary antibodies of interest (CD31-FITC, CD68-APC, and αSMA-Cy3) into the blocking buffer at a dilution of 1:100
CD31-FITC: stock concentration 0.5 mg/mL ; final working concentration 5 μg/mL.
CD68-APC: stock concentration 0.2 mg/mL ; final working concentration 2 μg/mL.
αSMA-Cy3: stock concentration 1.0–1.5 mg/mL; final working concentration 10-15 μg/mL.
-
68.
Carefully pipet the primary antibodies onto the tissue, pipetting enough of the solution to cover the tissue, roughly 200–300 μL
Note: This amount will vary depending on how large the tissue section is or the hydrophobic barrier enclosing the tissue
-
69.
Allow humid staining tray containing slides to remain in a cold room overnight for incubation (∼12–16 h)
Washes
Timing: 15 min
This section describes washing the microscope slides to reduce background signal.
-
70.
Wash slides three times in PBS 1X for 5 min
Secondary antibody incubation
Timing: 2 h
This section describes adding secondary antibodies. This is only necessary if the primary antibodies of interest are unconjugated. The antibodies listed in this protocol are conjugated and do not require secondary antibodies. Secondary antibodies should not be overlapping fluorophores and primary antibodies will need to be from different source animals to ensure no cross reactivity of secondary antibodies.
-
71.
Dilute secondary antibodies 1:250–1:500 into blocking buffer
Note: This should be done away from direct light
-
72.
Incubate slides for 2 h at room temperature (25°C)
Washes
Timing: 15 min
This section describes washing the microscope slides to reduce background signal.
-
73.
Wash slides three times in PBS 1X for 5 min
Sealing slides and imaging
Timing: 30 min
This section describes sealing the stained microscope slides with a cover slip to protect the stained tissue and imaging the final results.
-
74.
Use a dropper to apply a drop of DAPI in mounting media upon the section. Refer to Troubleshooting Problem 5 if you see air bubbles on the tissue
Note: In this protocol, we used DAPI in mounting media (see key resources table), so we did not need an additional mounting buffer. If another antibody is used, you may need to use a corresponding mounting buffer.
-
75.
Flat mount the slides with a cover slip
-
76.
Seal the edges of the slide using clear nail polish to preserve the sample
-
77.
Allow to dry for at least 30 min before use
-
78.
Image slides under microscope capable of immunofluorescence (e.g., Nikon Eclipse Ni-E) (see Figure 30 for example of immunofluorescence staining outcomes)
Note: Slides should be imaged within 2–3 weeks of staining for optimal results
Note: Refer to Troubleshooting Problem 6 for trouble with interpreting unexpected or residual staining
-
79.
Store dried slides at 4°C in a covered dry slide box
Figure 30.
Immunofluorescence micrographs of ischemic muscle tissue at Day 3 post femoral artery ligation from control or myeloid IL-1 -deleted mice (mIL-1 KO) mice (blue, DAPI; green, CD31; red, CD68; magenta, SMA)
Bar, 100 microns
Expected outcomes
A 98% success rate during HLI is expected in our experience. The majority of failures would be due to vessel rupture, thrombosis, hemorrhage, and death up to post-operative Day 3. Animals that appear healthy after three days are expected to survive throughout the full 21 days. There may be differences in survival in older animals or animals with predisposed disease states, such as obesity, diabetes, and atherosclerosis. Because of sterility of tools and careful, sterile surgical technique, our mice do well with the HLI procedure and laser Doppler imaging.
Limitations
While this experimental model of peripheral artery disease is able to induce hindlimb ischemia to assess blood flow recovery over 21 days, there are some limitations to this protocol. In modeling blood flow recovery by laser Doppler imaging, there can be discrepancies due to varying temperatures, positioning of the limbs or obstruction of the paws by fur, and sedation of the animal past the suggested time, leading to hypotension. In visualizing the vascular growth, while laser Doppler demonstrates blood flow recovery, microCT may be another method of visually mapping out new vessels within the muscle. Through immunofluorescence, we are able to demonstrate the recruitment of endothelial cells at Day 3, which serves as a surrogate for new blood vessels forming in the muscle tissue, as well as the underlying arteries using αSMA. However, to correlate the recruitment of endothelial cells with the density of mature vascular growth, immunofluorescence using these same markers must also be done on the muscle tissue at Day 21. There is some debate as to the choice of various antibody markers; von Willebrand Factor (VWF) in place of CD31, F4/80 or CD115 in place of CD68. VWF and CD31 both stain for endothelial cells, however, CD31 seems to have greater specificity for vascular differentiation. F4/80 demonstrates monocytes/macrophages, but F4/80 may not stain as well for tissue macrophages in comparison to monocytes circulating in the bloodstream. There has been some discussion regarding CD68 co-staining for neutrophils and some subsets of T-cells, however, this was shown to be very low (<2% of cells) when we carried out a clodronate liposome depletion experimental model and stained the gastrocnemius tissue using DAPI and CD68 in muscle tissue from mice expressing red fluorescent macrophages (Ai9 RCL-TdTomato Cre recombinase reporter strain).1
Troubleshooting
Femoral artery ligation
Problem 1
Difficulty identifying femoral artery (step 17)
Potential solution
Before making the first incision, identify landmarks. The inguinal ligament rests just below the level of the iliac crest. Try to identify other landmarks described above. If these are not identifiable, the incision may be made in the incorrect location. Though not ideal, you may need to make another or wider incision, and these should be closed appropriately with additional wound clips and monitored post-surgery.
Problem 2
Excessive bleeding of femoral vessel(s) suggesting perforation of the femoral vein or femoral artery (step 17)
Potential solution
Apply direct pressure using a sterile cotton swab over the area of hemorrhage.
If the femoral vein was perforated or torn, blood will pool and appears darker in color. With enough time, direct pressure via cotton swab may be adequate for hemostasis. If blood continues to pool, place a suture the end and ligate the artery at either the proximal or distal end. This will reduce the bleeding immediately from the femoral vein.
If the femoral artery was perforated or torn, the bleeding will not stop, will be pulsatile in nature and blood will be bright red. At this point, you should not continue the procedure, and immediate euthanasia should be performed.
Laser Doppler imaging
Problem 3
Static noise/random blue flecks around the limbs (step 42)
Potential solution
The heat lamp should be turned off while the scan is running. If you see blue flecks around the scan, the heat lamp may still be on during the Doppler scan. However, the heating pad should remain on during the scan. The heat lamp should be turned on immediately after the scan to support animal care and recovery.
Problem 4
Difficulty with visualizing blood flow in paws (step 43)
Potential solution
Before scanning the hindlimb, ensure that the limbs are stretched, the paws are flattened, and the tail is lying straight above the rectal probe. This will ensure adequate scanning and visualization of the blood flow into the entire paw. It is critical that the mouse reaches a temperature of 37 ± 0.5°C in order to accurately assess blood flow recovery.
Immunofluorescence staining
Problem 5
Air bubbles over the tissue (step 74)
Potential solution
The DAPI in multi-media should be carefully and sparingly dropped onto the tissue. When sealing the slide with a cover slip, it is important to place the cover slip from one side to the next, tapping out any potential air bubbles without sliding or damaging the tissue.
Problem 6
Specks of non-specific antibody on slide (step 78)
Potential solution
Antibodies should be centrifuged at 200 g for 10 min at 4 before use. Antibodies should never be vortexed, vigorously mixed, or pipetted up/down, as this can result in shearing of the antibody. Additionally, all three washes are necessary to wash away any excess antibody on the tissue.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Alan R. Morrison (alan_morrison@brown.edu).
Technical contact
Technical questions should be directed to and will be fulfilled by the technical contact, Chris Mantsounga, PhD (chris_mantsounga@brown.edu).
Materials availability
C57BL/6J (Cat# 000664), Csf1rmercremer (Cat# 019098), IL-1R−/− (Cat# 003245), and Ai9 (RCL-tdT) (Cat# 007909), male and female mice, aged 10–12 weeks, were obtained from The Jackson Laboratory. IL1βfl/fl mouse lines generated in this study will be available for purchase from The Jackson Laboratory or upon request to the lead contact through use of a material transfer agreement (MTA) with Ocean State Research Institute, Inc. The MTA will be necessary to guide (1) general terms on how the mice will be used; (2) liabilities; (3) husbandry and shipping costs; (4) prevention of inappropriate distribution; (5) appropriate acknowledgment of the source; and (6) insurances that all planned animal subjects use is compliant with NIH and VA regulations.
Data and code availability
This methods paper does not report any original code. Please refer to Mantsounga et al.1 for full data analyses. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
Research reported in this publication was supported by Research Project Grants NIH NHLBI R01HL139795 (A.R.M.), NIH 1R01HL163005 (A.R.M.), NIH NIGMS P20GM103652 (C.M. and A.R.M.), NIH 1R01HL163005-02S1 Diversity Supplement (O.C.), and NIH NIGMS 8P30 GM103410 (the Mouse Transgenic and Gene Targeting Facility at Brown University).
This work was also supported by VA VHA BLR&D 7IK2BX002527 (A.R.M.) and VA VHA CSR&D 1I01CX002231 (A.R.M.). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government. This work was also supported by an AHA Career Development Award AHA23CDA1056587 (C.M.) and Rhode Island Foundation #16415_139169 (C.M.).
Author contributions
A.R.M. conceived the study. S.S., C.M., and A.R.M. performed the in vitro and animal procedures. S.S., C.M., J.P., C.A.B., O.C., and A.R.M. analyzed the data. S.S., C.M., J.P., C.A.B., O.C., and A.R.M. wrote the protocol manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2025.103783.
Contributor Information
Chris Mantsounga, Email: chris_mantsounga@brown.edu.
Alan R. Morrison, Email: alan_morrison@brown.edu.
Supplemental information
Remove hair surrounding the surgical site that is 50% greater than the length of the incision. Nair should be kept on for approximately 45 s and wiped away with moistened gauze, followed by sterile water washes to prevent any chemical burns.
Using blunt serrated forceps, gently pinch the skin at the level of the inguinal ligament. Make a 45-degree incision with dissecting scissors that extends 5 mm.
Use Dumont forceps to clean away the superficial white adipose tissue until the femoral artery, femoral vein, and femoral nerve are exposed. The femoral artery appears as a thin, pale pink band above a dark red vein, with the head of the vein appearing bulbous and large.
Isolate the femoral nerve away from the femoral artery and femoral vein by gently flexing open curved Dumont forceps.
Isolate the femoral artery from the femoral vein. Use 7-0 polypropylene sutures to ligate the proximal end of the femoral artery, just below the inguinal ligament.
Ligate the artery using an instrument tie.
Use dissecting scissors to trim the ends of the suture as closely as possible.
Use 7-0 polypropylene sutures to ligate the distal end of the femoral artery.
Ligate the artery using an instrument tie. Trim the sutures using dissecting scissors.
Flex curved Dumont forceps to ensure full separation of the femoral artery from the femoral vein.
Use Vannas scissors to excise the femoral artery between the two ligations.
Pinch the skin together using blunt serrated forceps. Using a wound clamp, secure the wound with a 9 mm wound clip.
Wipe the wound with betadine before moving the mouse to the recovery platform.
References
- 1.Mantsounga C., Lee C., Neverson J., Sharma S., Healy A., Berus J.M., Parry C., Ceneri N.M., López-Giráldez F., Chun H.J., et al. Macrophage IL-1β promotes arteriogenesis by autocrine STAT3- and NF-κB-mediated transcription of proangiogenic VEGF-A. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Research Council . Eighth Edition. The National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Perform a two-stage scrub on the hindlimb with betadine followed by 70% isopropyl alcohol.
Remove hair surrounding the surgical site that is 50% greater than the length of the incision. Nair should be kept on for approximately 45 s and wiped away with moistened gauze, followed by sterile water washes to prevent any chemical burns.
Using blunt serrated forceps, gently pinch the skin at the level of the inguinal ligament. Make a 45-degree incision with dissecting scissors that extends 5 mm.
Use Dumont forceps to clean away the superficial white adipose tissue until the femoral artery, femoral vein, and femoral nerve are exposed. The femoral artery appears as a thin, pale pink band above a dark red vein, with the head of the vein appearing bulbous and large.
Isolate the femoral nerve away from the femoral artery and femoral vein by gently flexing open curved Dumont forceps.
Isolate the femoral artery from the femoral vein. Use 7-0 polypropylene sutures to ligate the proximal end of the femoral artery, just below the inguinal ligament.
Ligate the artery using an instrument tie.
Use dissecting scissors to trim the ends of the suture as closely as possible.
Use 7-0 polypropylene sutures to ligate the distal end of the femoral artery.
Ligate the artery using an instrument tie. Trim the sutures using dissecting scissors.
Flex curved Dumont forceps to ensure full separation of the femoral artery from the femoral vein.
Use Vannas scissors to excise the femoral artery between the two ligations.
Pinch the skin together using blunt serrated forceps. Using a wound clamp, secure the wound with a 9 mm wound clip.
Wipe the wound with betadine before moving the mouse to the recovery platform.
Data Availability Statement
This methods paper does not report any original code. Please refer to Mantsounga et al.1 for full data analyses. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.