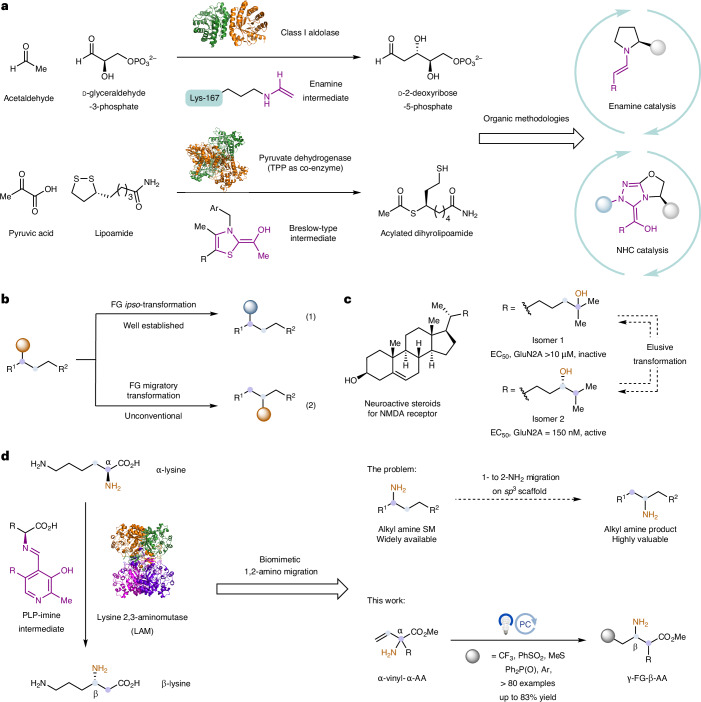
Fig. 1. Biomimetic 1,2-amino migration via photoredox catalysis.
a, The development of new organic methods is often inspired by enzymatic transformation. b, FG migratory transformation is a valuable but underdeveloped strategy for molecular modification, especially for late-stage functionalization. c, Regioisomers often pose quite different properties in drug discovery. d, One particular type of FG migratory transformation that intrigued us is 1,2-amino migration, when an amino group is translocated to its neighbouring location on an sp3 scaffold. Given the abundance of alkyl amines as feedstock reagents and their importance as synthetic building blocks, we believe that the chemical community could greatly benefit from such a method. However, to our knowledge, 1,2-amino migration has not yet been established as a practical technique in organic chemistry. Here we have developed a biomimetic photoredox-catalysed 1,2-amino migration method. This method was inspired by a LAM-catalysed α-lysine to β-lysine transformation. AA, amino acid; TPP, thiamine pyrophosphate; NHC, N-heterocyclic carbene; NMDA, N-methyl-d-aspartate; EC50, half maximal effective concentration.