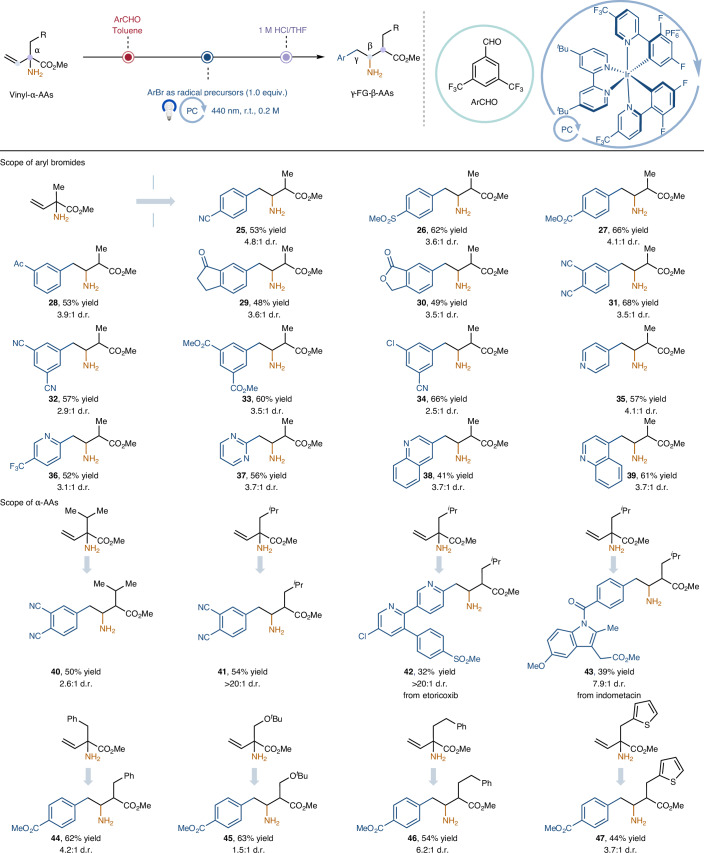
Table 2.
Synthesis of γ-aryl-β-amino acids via 1,2-amino migration
See Supplementary Section 4.5 for full experimental details. All yields are isolated yields in their hydrochloride salt forms except for products 43 and 45, which were isolated in their free amine forms. Reaction condition for the 1,2-amino migration step: 2 (1.5 mmol), aryl bromides (0.3 mmol), Ir[dF(CF3)ppy]2(dtbbpy)PF6 (1 mol%), K2CO3 (2.0 equiv.), (TMS)3SiNH-Ad (1.0 equiv.), MeCN (1.5 ml), λmax = 440 nm Kessil (40 W), N2, r.t., 2 h, then work-up with 1 M HCl in THF, 6 h; isolated yield over two steps from vinyl-α-aldimine esters. TMS, trimethylsilyl; Ad, adamantly.