FIG. 7.
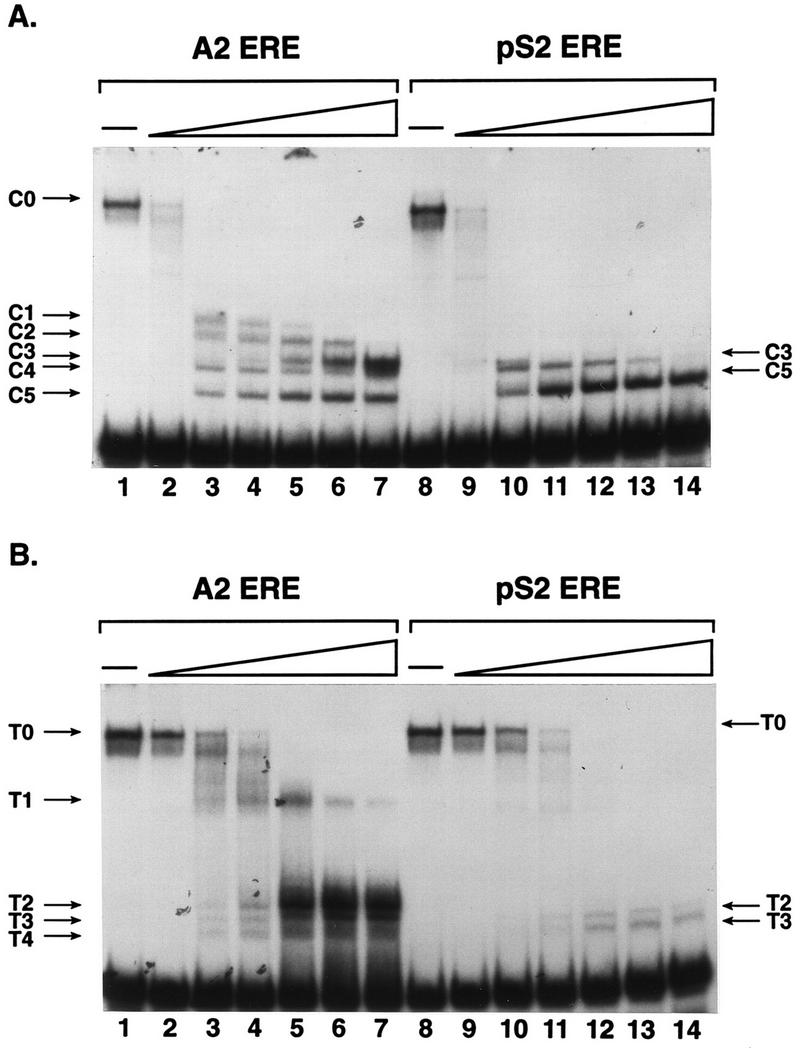
Distinct protease digestion patterns of A2 and pS2 ERE-bound ER provide evidence for ERE-mediated differences in receptor conformation. (A) Partially purified, estrogen-occupied ER was combined with A2 or pS2 ERE-containing DNA fragments. After a short incubation, 0, 0.05, 0.5, 1.25, 2.5, 3.75, or 5 ng of chymotrypsin was added to the binding reaction. ER-DNA complexes and free DNA were fractionated on a nondenaturing acrylamide gel, and the gel was dried and subjected to autoradiography. The undigested ER-DNA complex (C0) and ER-DNA complexes formed with chymotrypsin-proteolyzed receptor (C1 to C5) are indicated. (B) Partially purified ER and A2 or pS2 ERE-containing DNA fragments were combined as for panel A except that 0, 0.05, 0.5, 1.25, 2.5, 3.75, or 5 ng of trypsin was added to the binding reactions. The undigested ER-DNA complex (T0) and ER-DNA complexes formed with trypsin-proteolyzed receptor (T1 to T4) are indicated.
