Abstract
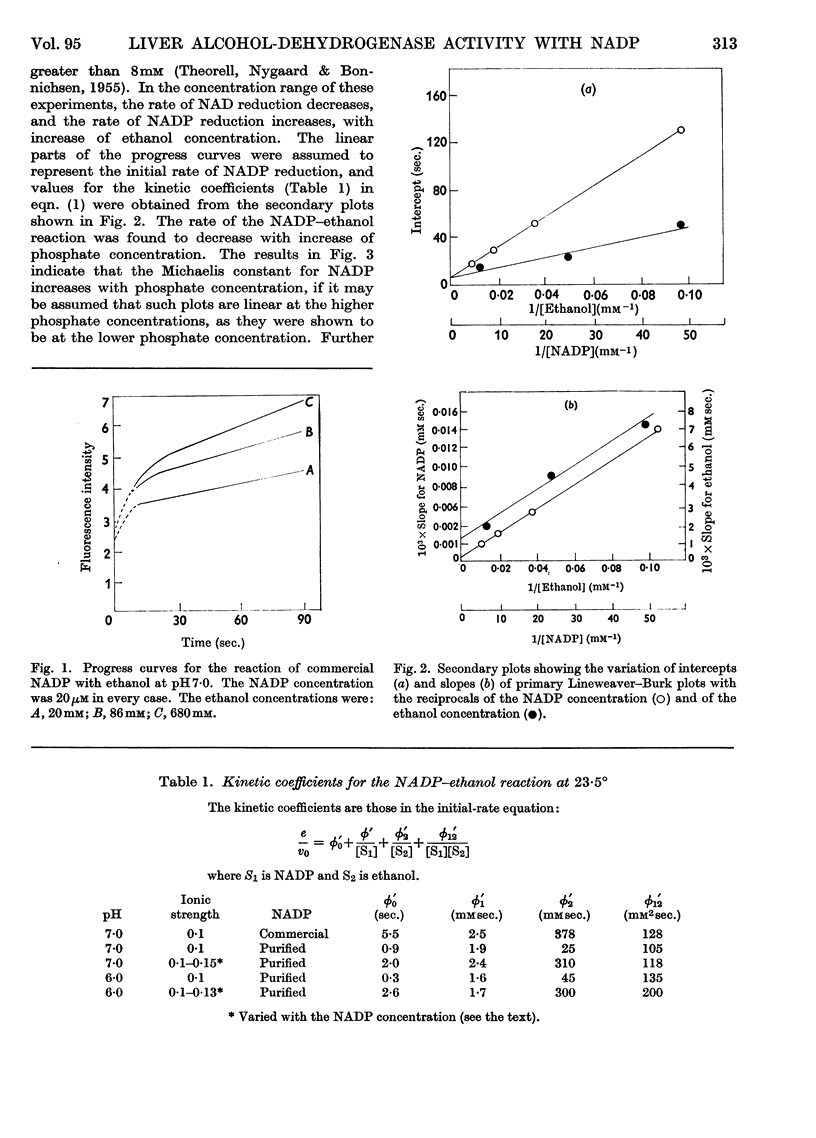
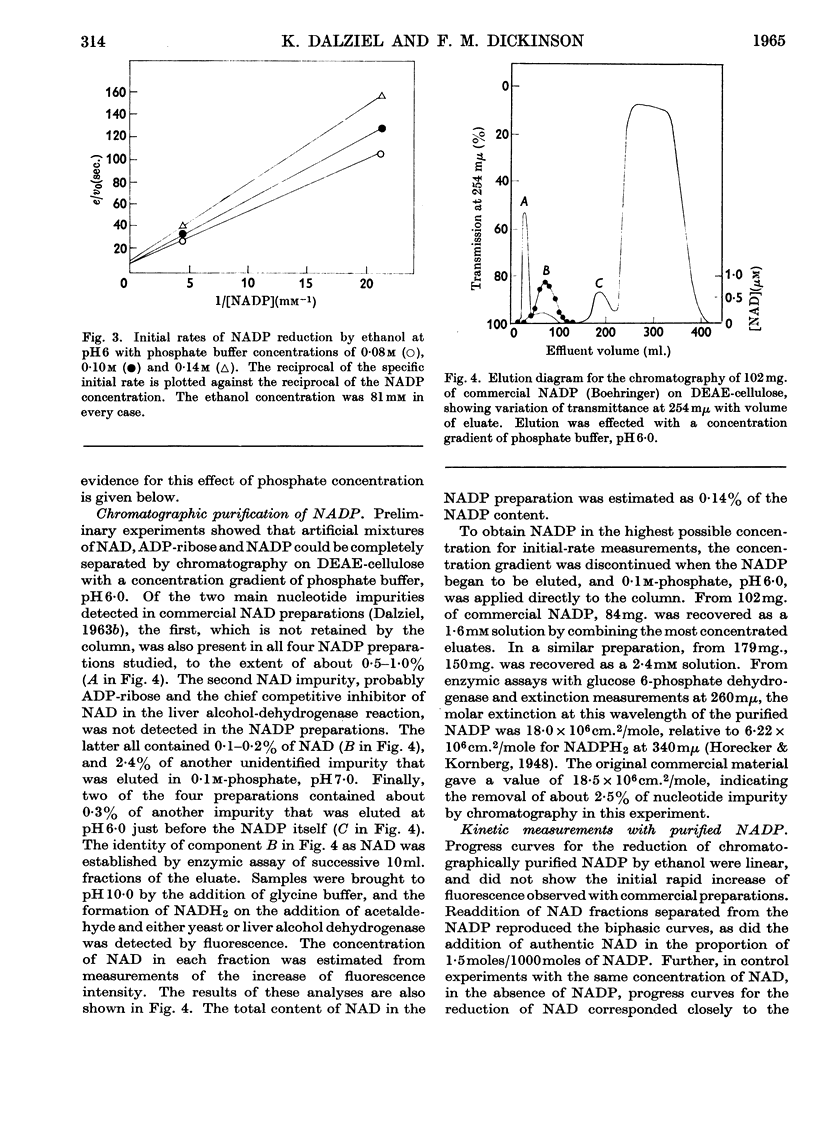
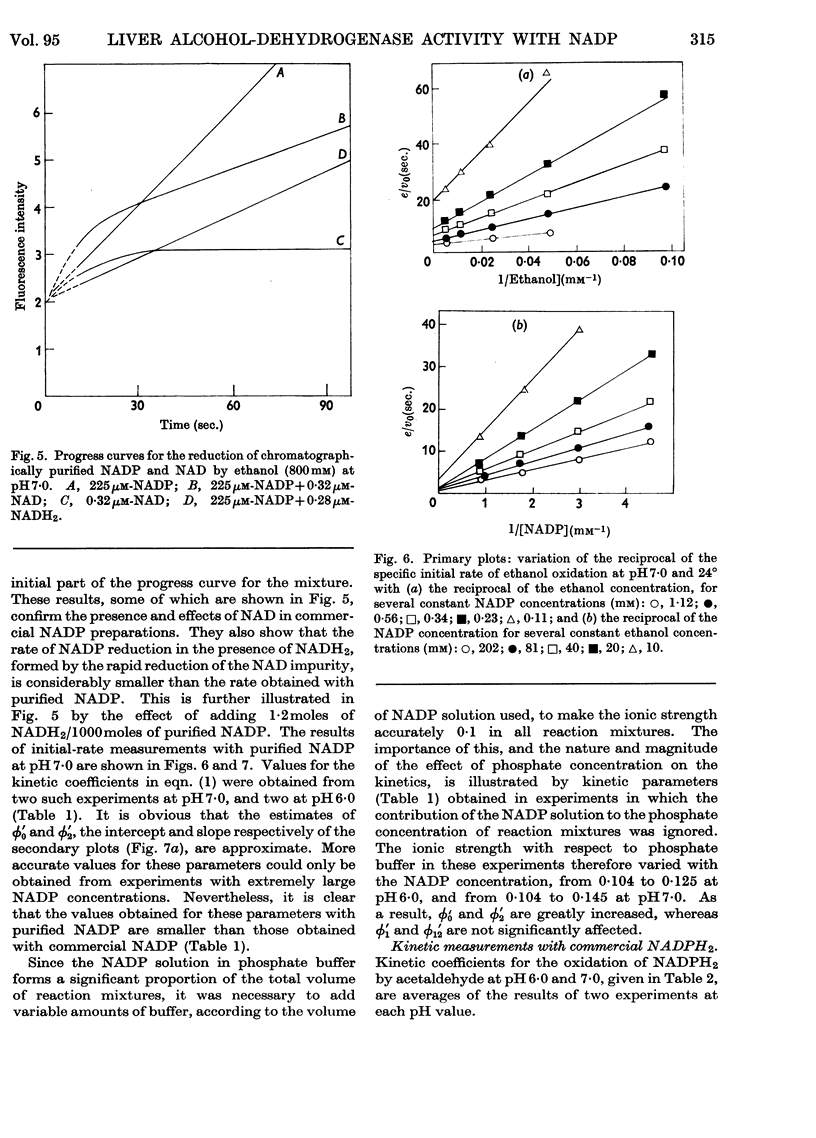
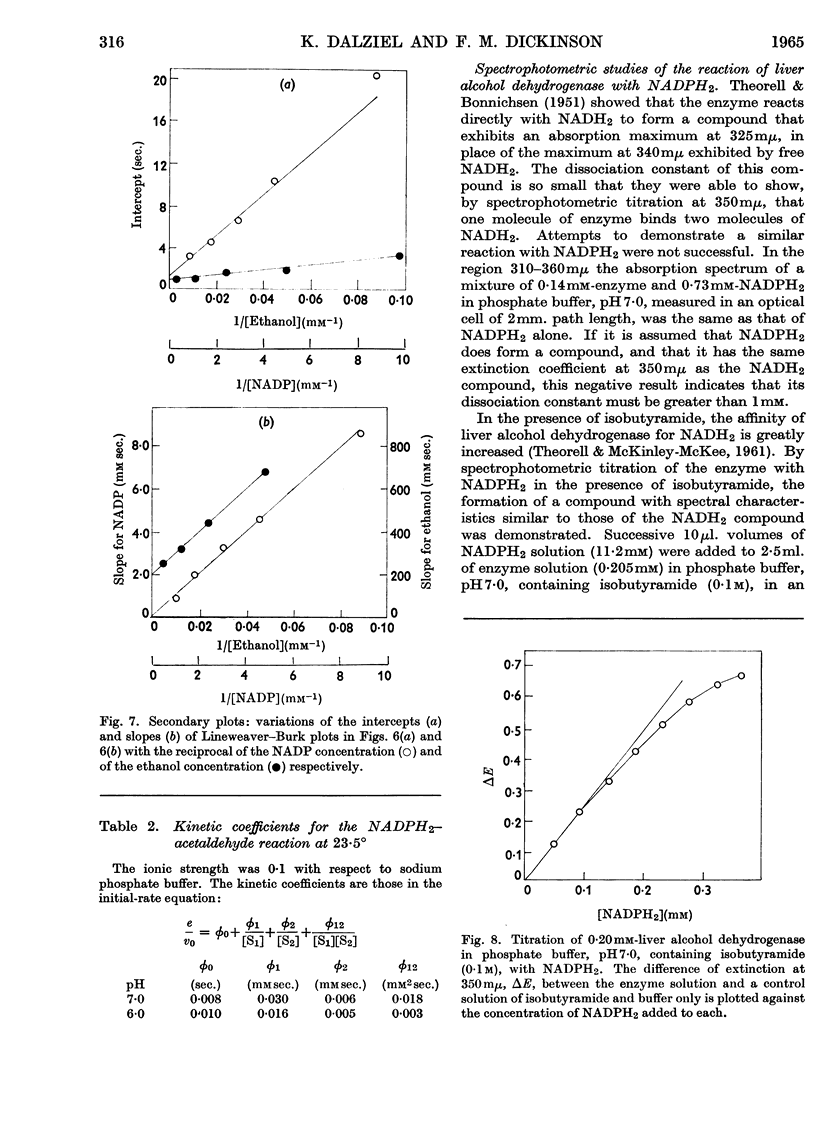
1. The separation of nucleotide impurities from commercial NADP preparations by chromatography is described. All the preparations studied contained 0·1–0·2% of NAD. 2. The activity of pure crystalline liver alcohol dehydrogenase with NADP as coenzyme has been confirmed. Initial-rate data are reported for the reaction at pH 6·0 and 7·0 with ethanol and acetaldehyde as substrates. With NADP and NADPH2 of high purity, the maximal specific rates were similar to those obtained with NAD and NADH2, but the Michaelis constants for the former coenzymes were much greater than those for the latter. 3. The oxidation of ethanol by NADP is greatly inhibited by NADH2, and this accounts for low values of certain initial-rate parameters obtained with commercial NADP preparations containing NAD. The kinetics of the inhibition are consistent with competitive inhibition in a compulsory-order mechanism. 4. Initial-rate data with NAD and NADPH2 do not conform to the requirements of the mechanism proposed by Theorell & Chance (1951), in contrast with results previously obtained with NAD and NADH2. The possibility that the deviations are due to competing nucleotide impurity in the oxidized coenzyme cannot be excluded. The data show that the enzyme reacts more slowly with, and has a smaller affinity for, NADP and NADPH2 than NAD and NADH2. 5. Phosphate behaves as a competitive inhibitor towards NADP.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DALZIEL K. An inhibitor of liver alcohol dehydrogenase in preparations of reduced diphosphopyridine nucleotide. Nature. 1961 Sep 9;191:1098–1099. doi: 10.1038/1911098a0. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. KINETIC STUDIES OF LIVER ALCOHOL DEHYDROGENASE AND PH EFFECTS WITH COENZYME PREPARATIONS OF HIGH PURITY. J Biol Chem. 1963 Aug;238:2850–2858. [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. Possible magnitude of inhibition of coenzyme-substrate reactions by competitive inhibitors in coenzyme preparations. Nature. 1962 Jul 28;195:384–385. doi: 10.1038/195384a0. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. Some observations on the preparation and properties of dihydronicotinamide-adenine dinucleotide. Biochem J. 1962 Aug;84:240–244. doi: 10.1042/bj0840240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. The preparation and properties of crystalline alcohol dehydrogenase from liver. Biochem J. 1961 Aug;80:440–445. doi: 10.1042/bj0800440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- PULLMAN M. E., COLOWICK S. P., KAPLAN N. O. Comparison of diphosphopyridine nucleotide with its deaminated derivative in various enzyme systems. J Biol Chem. 1952 Feb;194(2):593–602. [PubMed] [Google Scholar]


