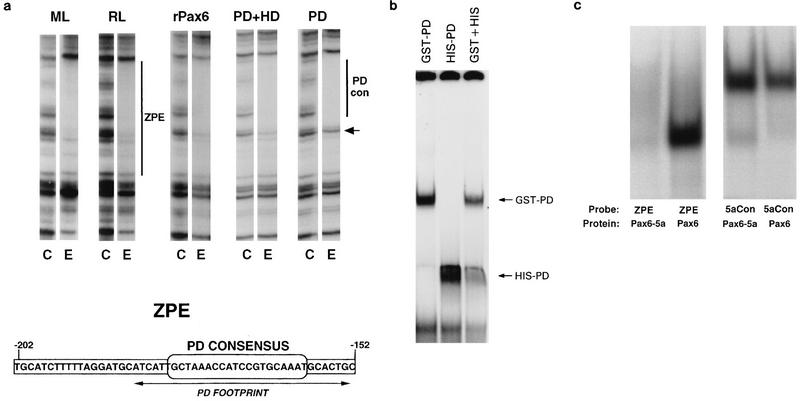
FIG. 1.
Binding of canonical Pax6 to the ZPE. (a) Binding of lens cell extracts and recombinant Pax6 proteins to the ζ ZPE. DNase I footprints of the upper strand of the ZPE region are shown. Pairs of lanes show results for control with no added protein (C) and a representative lane for addition of lens cell nuclear extract or recombinant proteins (E). For each experiment, DNase I and protein concentrations were titrated, but for clarity only a single representative lane for each protein is shown. In each case, two- to fivefold increases in the DNase I concentration or twofold increases in the protein concentration gave no difference in protection. Extracts and proteins were as follows: ML, mouse lens-derived αTN4-1 cells; RL, rabbit lens N/N1003A cells; rPax6, full-length human Pax6; PD+HD, a truncated Pax6 containing PD and HD; PD, Pax6 PD. The small arrow shows a band which is efficiently protected by full-length rPax6 but not by PD alone. Bars show the maximal extent of the ZPE (−202 to −152) and the region corresponding to the Pax6 PD consensus binding site (−184 to −152). The sequence of the ZPE region of the ζ promoter is shown below. The position of the consensus PD binding sequence is indicated. Arrows show the extent of the experimental protection due to PD alone, which overlaps the PD consensus. (b) EMSA of the ZPE (−202 to −152) with two different-size Pax6 PD fusion proteins, GST-Pax6 and His-Pax6. The combination of the two forms produced no intermediate-size shifts. (c) The ZPE (−202 to −152) binds canonical Pax6 but not Pax6-5a. Results are shown for EMSA of the labelled ZPE and for 5aCon (a different sequence derived in vitro as a Pax6-5a binding sequence [7]), using recombinant Pax6 and Pax6-5a proteins as shown.