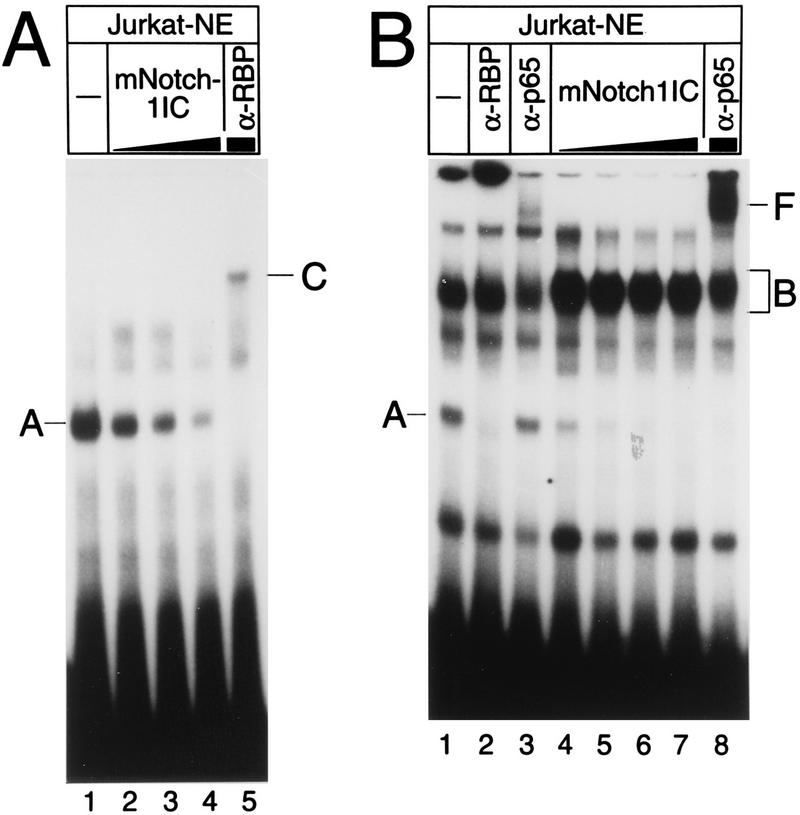
FIG. 6.
Cell-free system-synthesized Notch-1-IC interacts with Rep-κB/RBP-Jκ from Jurkat T cell nuclear extracts (Jurkat-NE) at the HES-1 and NF-κB2 promoters. (A) Extracts (2 μg) were incubated without (lane 1) or with increasing amounts of (lanes 2 to 5) reticulocyte lysate programmed with Notch-1-IC (mNotch-1IC). Volumes were equalized by addition of pure reticulocyte lysate. A 32P-labeled HES-1-specific double-stranded oligonucleotide (SL366) was used as a probe. Rep-κB/RBP-Jκ binding activity (complex A) is evidenced by an RBP-Jκ-specific antibody (α-RBP) supershifting a novel band (complex C). Note that this more slowly migrating band (lane 5) does not represent a Notch-1-IC-containing complex. (B) Extracts (2 μg) were incubated without (lane 1) and with increasing amounts of (lanes 4 to 8) reticulocyte lysate programmed with Notch-1-IC. 32P-labeled NF-κB2-specific oligonucleotide SL332 was used as a probe. In addition to the Rep-κB/RBP-Jκ-specific DNA binding activity (complex A) that was recognized by a specific antibody (α-RBP), a second complex (complex B) was detected in nuclear extracts and was more abundant after addition of reticulocyte lysate. Treatment with an antiserum directed against RelA (p65) (α-p65) interfered with this binding activity and supershifted band F (lanes 3 and 8).