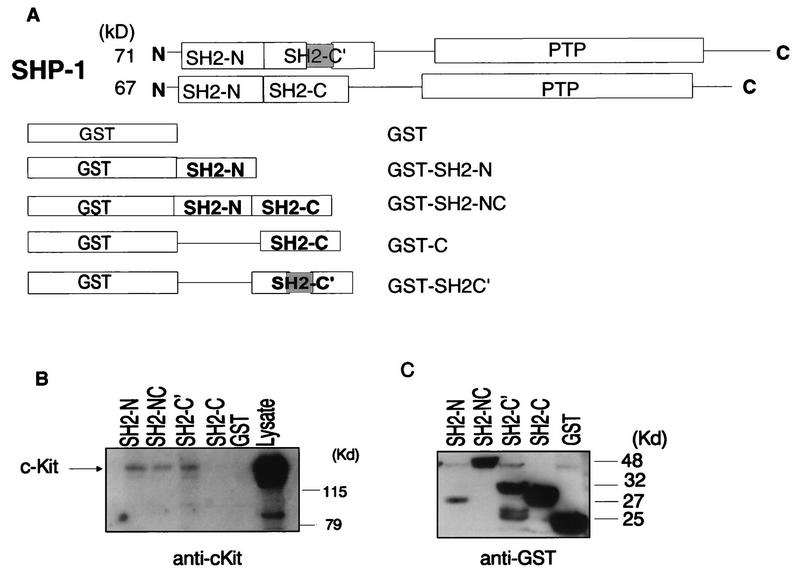
FIG. 2.
The Kit receptor preferentially associates with the SHP-1 SH2-N and SH2-C′ domains. (A) Schematic diagram showing the structures of the 71- and 67-kDa splice variants of SHP-1 and the SHP-1 SH2 domain sequences present in the GST–SHP-1 fusion proteins used for in vitro binding assays. The shaded region represents a 39-amino-acid segment present in the C-terminal SH2 domain (SH2-C′) of the 71-kDa, but not the 67-kDa, SHP-1 species. (B and C) Cell lysates (1,800 μg) prepared from 108 SCF-stimulated HEL cells were incubated for 2 h at 4°C with 5 μg of purified GST–SHP-1 fusion protein immobilized on glutathione-Sepharose beads. Complexes as well as lysate alone were fractionated by SDS-PAGE and subjected to immunoblotting with anti-c-Kit (B) or anti-GST (C) antibodies. Mobilities of molecular mass (MW) standards are shown on the right.