Abstract
Background
Near-infrared fluorescence (NIRF) imaging assists surgeons intraoperatively to achieve radical resection of malignant tissue with one centimeter depth and can be supplemented with photoacoustic imaging to increase depth-of-view. Tumor-associated carbohydrate antigens are promising targets for tumor imaging with potential advantages over protein targeting. This study preclinically evaluates the anti-glycan tracers CH88.2-800CW (anti-Lea/c/x) and CH129-800CW (anti-sdi-Lea) for bimodal NIRF/PA imaging of gastrointestinal cancers.
Results
Using immunohistochemistry, we found that Lea/c/x and sdi-Lea were highly expressed in gastric and colorectal cancer tissue, with limited expression in healthy surrounding tissue, except for strong Lea/c/x expression in healthy colorectal epithelium. Bimodal NIRF/PA imaging using CH88.2-800CW and CH129-800CW was performed on subcutaneous and orthotopic HT-29_luc2 (colon cancer) and BxPC-3_luc2 (pancreatic cancer) tumor-bearing mice, using rituximab-800CW as a negative control tracer. At 96 h post-injection, all orthotopic tumors were delineated using the clinical Artemis NIRF imager with mean CH88.2-800CW and CH129-800CW tumor-to-background ratios of 4.8 ± 1.4 and 4.9 ± 0.5 for the HT-29_luc2 model, and 2.5 ± 0.3 and 2.9 ± 0.4 for the BxPC-3_luc2 model, respectively. Similarly specific photoacoustic signal was observed within all tumors for both CH88.2-800CW and CH129-800CW. Biodistribution analyses showed high tumor fluorescence with minimal signal in healthy organs, including the liver and kidneys.
Conclusions
Bimodal NIRF/PA imaging employing CH88.2-800CW and CH129-800CW facilitates real-time, high-contrast gastrointestinal tumor visualization. Given their strong and mostly tumor-specific expression, both tracers hold promise as effective imaging agents for gastrointestinal cancers, and are compelling candidates for further clinical evaluation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13550-025-01258-y.
Keywords: Fluorescence-guided surgery, Photoacoustic imaging, Carbohydrates, Gastrointestinal cancers
Background
Achieving local control through radical surgical resection remains the cornerstone of curative cancer treatment [1]. However, the widespread adoption of non-invasive surgical techniques, such as laparoscopy and robotics, has reduced the surgeon’s ability for tumor identification by direct tactile feedback. Fortunately, novel techniques facilitating intraoperative identification of tissues-of-interest have emerged, such as near-infrared fluorescence (NIRF) imaging, also known as fluorescence-guided surgery. This method allows real-time visualization of tissue using a NIRF contrast agent that is visualized through a dedicated camera system [2, 3]. Its potential in distinguishing malignant tissue from healthy surrounding tissue intraoperatively has been widely demonstrated across various tumor types in both the preclinical and clinical setting [3, 4].
Precise alignment of the NIRF imaging tracer with the tumor type of interest remains a key prerequisite for adequate tumor visualization. As most of the current target-tracer combinations appeared to have their shortcomings, including target expression in healthy surrounding tissues, heterogeneity of target expression and an unsuitable tracer clearance profile, the quest for novel targets continued. Previous work by our group has proposed tumor-associated carbohydrate antigens (TACAs) as an alternative and promising class of tumor-specific targets for NIRF imaging of cancer, with the potential to overcome aforementioned limitations [5–8].
Aberrant glycosylation of proteins and lipids is a major characteristic of cancer [9]. This results in, among others, the appearance of complex Lewis glycan variants as well as a strong overexpression of Lewis glycans normally expressed in healthy tissue. Most of these TACAs, including sialyl-Lewisa (also known as CA19-9) and sialyl-Lewisx, play crucial roles in tumor progression, both directly and indirectly by adapting their carrier’s configuration. Considering their strong (over)expression in the outmost layer of the cell membrane and on multiple tumor-associated proteins, TACAs possess unique characteristics that make them ideal targets for NIRF imaging [10–13].
Chua and Tivadar et al. described two monoclonal antibodies (mAbs), CH88.2 and CH129, targeting unique sets of Lewis glycan epitopes, Lewisa/c/x (Lea/c/x) and sialyl-di-Lewisa (sdi-Lea), respectively [12, 13]. These targets have been found to be highly-specific for human epithelial cancers, with limited expression in healthy tissue. Our group confirmed the abundance of Lea/c/x (83%) and sdi-Lea (94%) in human pancreatic ductal adenocarcinoma (PDAC) tissue specimens, as well as in tumor-positive lymph nodes and PDAC fine-needle biopsies [7]. Notably, both biomarkers demonstrated significantly lower expression in surrounding healthy pancreatic tissue and chronic pancreatitis compared to PDAC tissue, establishing both targets as suitable for NIRF imaging [7, 8]. In a proof-of-concept in vivo study, NIRF imaging using CH88.2-800CW provided high-contrast tumor delineation at 96 h post-injection in human colon and pancreatic xenograft models using both preclinical and clinical NIRF imagers, thereby establishing the preclinical potential of the tracer for NIRF imaging of cancer [6].
While NIRF imaging effectively visualizes superficial lesions, its application is less suitable for deeper-located lesions due to the limited penetration depth of NIR light, namely ~ 7 mm [14]. One solution may be augmenting NIRF imaging with another real-time imaging modality, such as photoacoustic (PA) imaging.
PA imaging using ultrasound (US) detects acoustic waves excited by NIRF dyes following exposure with a nanosecond pulsed NIR laser [15]. The technique yields a higher spatial resolution than optical NIRF imaging and allows visualization of tissue up to 7 cm. Bimodal NIRF/PA imaging may synergistically improve tumor detection by supplementing superficial NIRF imaging with the enhanced “depth-of-view” of PA-imaging, using a single tracer administration [16, 17].
Building upon previous work into glycan-targeted tumor imaging, the current study presents the extensive preclinical evaluation of anti-Lewis glycan tracers CH88.2-800CW and its counterpart CH129-800CW for bimodal NIRF/PA imaging of cancer. To accomplish this, expression of their targets, Lea/c/x and sdi-Lea, respectively, is first verified in gastrointestinal malignancies using immunohistochemistry on human tissue specimens of gastric and colorectal cancer, as well as healthy surrounding tissue. Of note, the abundant and tumor-specific expression of both epitopes in pancreatic cancer has been described elsewhere [7]. Thereafter, binding of CH88.2-800CW and CH129-800CW is evaluated both in vitro followed by in vivo in human tumor xenograft mouse models.
Materials & methods
Monoclonal antibodies
Anti-Lea/c/x and anti-sdi-Lea chimeric mAbs CH88.2 (hIgG1) and CH129 (hIgG1) and their murine derivates FG88.2 (IgG3) and FG129 (IgG1k) were kindly supplied by prof. Lindy Durrant (Scancell Ltd, UK) and produced as described elsewhere [12, 13].
Conjugation of monoclonal antibodies
CH88.2 and CH129 were covalently conjugated with IRDye 800CW using N-hydroxysuccinimide (NHS)-ester chemistry against primary amino groups until a degree of labeling of 1-1.5 was reached, following the manufacturers protocol (LI-COR, Lincoln, NE, Nebraska, USA). Degrees-of-labeling were quantified through photo spectrophotometry and confirmed using MALDI-TOF analyses. Conjugation results were evaluated using SDS-PAGE on 4–20% protein gels (Criterion, Bio-Rad laboratories, Veenendaal, The Netherlands). Proteins were stained using Coomassie brilliant blue G-250 (Bio-Rad laboratories). Fluorescence images of the gel were acquired using the Odyssey CLx Infrared Imaging System (LI-COR) with the 800 nm channel.
Patient selection and specimen selection
Representative formalin-fixed paraffin-embedded tissue blocks of patients diagnosed with gastric (n = 52) or colorectal (n = 36) cancer were obtained. Colorectal and gastric tissue blocks were obtained from the department of Pathology of the Leiden University Medical Center (The Netherlands). Tissue blocks contained tumor tissue and were particularly selected to contain adjacent normal tissue. Clinicopathological data were obtained from the patients’ medical records. The research protocol received approval from both the Gastroenterology Biobank Review Committee (protocol reference: 2020-16) and the local Medical Ethical Review Committee (protocol reference: B20.052. The study strictly adhered to the Dutch code of conduct for responsible use of human tissue in medical research. All tissue specimens and associated clinicopathological data were utilized in an anonymized manner and in accordance with the principles outlined in the Declaration of Helsinki (1964).
Immunohistochemistry
Immunohistochemistry was performed as extensively described elsewhere [7]. Briefly, 4-µm-thick sections were deparaffinized, rehydrated, followed by endogenous peroxidase blocking and antigen retrieval by heating sections in EnVision Flex Target Retrieval Solution (pH 6.0). Sections were incubated overnight with FG88.2 (0.19 µg/ml) or FG129 (0.12 µg/ml) or a pan-cytokeratin-directed antibody (AE1/AE3, Agilent Technologies, Inc., Santa Clara, CA, USA). Slides were incubated with secondary anti-mouse EnVision antibodies (Dako, K4001); staining was visualized using DAB (K3468, Agilent), followed by counterstaining with Mayer’s hematoxylin solution and drying. Histological reference slides were stained with Mayer’s hematoxylin solution and counterstained with eosin. All slides were mounted with Pertex, digitized using the panoramic digital slide scanner and analyzed using CaseViewer 2.4 (both 3D Histech, Budapest, Hungary).
Evaluation of immunohistochemical staining
Immunohistochemical membranous staining on malignant and healthy tissue was quantified using the total immunostaining score (TIS), which is calculated by multiplying the staining proportion (0 = ≤ 9%, 1 = 10–25%, 2 = 26–50%, 3 = 51–75%, 4 = ≥ 76%) by the staining intensity (0 = none, 1 = weak, 2 = moderate, 3 = strong). A categorical TIS was constituted as follows: 0 = negative; 1, 2, 3, 4 = weak expression; 6, 8 = moderate expression; 9, 12 = strong expression. Scoring was performed by three independent observers (RH, MvD and ASLPC). Samples without agreement were discussed in a consensus meeting, in which the final score was determined.
Human cancer cell lines
Cell lines HT-29_luc2, COLO-320, COLO 205, DLD-1 (colon carcinoma), PANC-1, and MIA PaCa-2 (pancreatic carcinoma) were obtained from ATCC, while BxPC-3_luc2 was purchased from PerkinElmer (Waltham, MA, USA). HT-29, DLD-1, COLO-320 COLO-205, and BxPC-3(_luc2) cells were cultured in RPMI 1640 cell culture medium (Gibco, Invitrogen, Carlsbad, CA, USA). PANC-1 and MIA Paca-2 were cultured in DMEM + GlutaMAX™ cell culture medium (Gibco, Invitrogen). The absence of Mycoplasma contamination was confirmed using polymerase chain reaction (PCR) analysis. Cell cultures were maintained in a humidified incubator set at 37 °C with 5% CO2, and upon reaching 90% confluence, cells were detached using trypsin/EDTA (0.5% Trypsin-EDTA solution 10×, obtained from Santa Cruz Biotechnology, Inc, Dallas, TX, USA). Viability assessments were conducted using trypan blue staining in a 0.4% solution (Invitrogen).
Cell-based plate assay
Binding of CH88.2-800CW and CH129-800CW was evaluated on colon carcinoma cell lines HT-29_luc2, COLO-320, COLO 205, DLD-1 and pancreatic cancer cell lines BxPC-3_luc2, PANC-1, and MIA PaCa-2 using cell-based plate assays. Cells were cultivated in a 96-well plate at a density of 20,000 cells per well in 100 µl of complete medium (Corning Costar Inc., Cambridge, MA, USA), until reaching 90% confluence. Subsequently, the cells were washed twice with PBS supplemented with 0.5% bovine serum albumin (0.5% PBSA). To assess CH88.2-800CW and CH129-800CW binding, cells were exposed to CH88.2-800CW or CH129-800CW in PBS at concentrations of 3, 6, 12, 25 50, or 100 nM, for 1 h, on ice and shielded from light. Following incubation, the cells were rinsed twice with 0.5% PBSA to eliminate any unbound tracer. The fluorescence emitted by CH88.2- or CH129-800CW was assessed using the Odyssey CLx Infrared Imaging System (LI-COR) with the 800 nm channel (excitation 785 nm, emission filter 812–823 nm). To estimate cell numbers through nuclear fluorescence, cells were permeabilized using 40–60% acetone-methanol for 5 min, washed, and then treated with ToPro-3 iodide (1:2000, T3605, Invitrogen, California, USA) for 10 min at room temperature. After another washing step, nuclear fluorescence was quantified using the 700 nm channel of the Odyssey (excitation 685 nm, emission filter 710–730 nm). The mean fluorescence intensity (MFI) was computed by dividing the 800-nm fluorescence signal by the nuclear 700-nm signal. All experiments were conducted in triplicate.
Chamber slides
Following detachment and viability assessment, cells were transferred to an 8-well Nunc™ Lab-Tek™ II Chamber Slide (0.7 cm2/well, Thermo Fisher Scientific) at a density of 40,000 cells per well. Upon achieving 90% confluence, the cell culture medium was aspirated, and the cells underwent two 5-minute washing steps with PBS. Subsequently, cells were fixed using 1% paraformaldehyde at room temperature for 10 min, followed by two 5-minute washes with PBS. The cells were then exposed to CH88.2-800CW, CH129-800CW or negative control tracer rituximab (anti-CD20)-800CW on ice (50 nM), shielded from light, for 1 h, after which they were washed with PBS and demineralized water. The plastic chambers were removed, and the slides were air-dried before staining with ProLong Gold containing DAPI (Thermo Fisher Scientific). Imaging of the slides was performed using the DAPI channel (excitation 376–398 nm, emission filter 417–477 nm) and the Cy7 channel (excitation 773–758 nm, emission filter 776–826 nm) of the Axio Scan Z1 (Carl Zeiss AG, Oberkochen, Germany). Image analysis was performed using Zen Lite software (version 3.5, Zeiss). All experiments were conducted in triplicate.
Animal models
Mice were housed at the Central Animal Facility of the LUMC, where they were maintained according to EU Recommendation 2007-526-EC guidelines under specific pathogen-free conditions All animal procedures strictly adhered to local standard operating procedures [18]. Female BALB/c-Nude (CAnN.Cg-Foxn1nu/Crl) mice, aged between six to eight weeks, were procured from Charles River Laboratories, Wilmington, MA, USA. For subcutaneous models, mice were subcutaneously injected at four locations on their backs with either HT-29_luc2 or BxPC-3_luc2 cells (500,000 cells/spot; n = 3 mice per group). Tumor growth was monitored using a digital caliper, and tumors reaching a volume of 50 mm3 were considered suitable for imaging. Orthotopic HT-29_luc2 and BxPC-3_luc2 models were induced as described elsewhere [19, 20]. Briefly, HT-29_luc2 tumors were subcutaneously grown, resected, fragmented and kept on ice. After performing a midline incision, the HT-29_luc2 fragment was attached to the cecum wall using a 6 − 0 Ethilon suture. For the BxPC-3 model, a left lateral flank incision was performed, after which 500,000 BxPC-3_luc2 cells (resuspended in 50 µl PBS) were injected into the body of the pancreas. Orthotopic tumors were grown for approximately two weeks and growth was monitored by bioluminescence imaging using the IVIS® Spectrum Preclinical In Vivo Imaging System (Spectrum, PerkinElmer, MA, USA). At the end of the experiments, mice were sacrificed using CO2. The animal studies underwent thorough review and approval by the local animal welfare body at the LUMC. Animals were cared for in accordance with the Code of Practice Animal Experiments in Cancer Research and guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. The handling of animals adhered to established local standard operating procedures.
In vivo NIRF imaging
Once subcutaneous tumors reached a volume of around 50 mm3, mice were administered either 1 nmol of CH88.2-800CW, CH129-800CW or rituximab-800CW dissolved in PBS via tail vein injection. In the case of orthotopic tumors, tumors emitting a bioluminescence signal exceeding 1.0 × 108 p/sec/cm2/sr were deemed appropriate for imaging. Imaging sessions for subcutaneous tumor-bearing mice were conducted at intervals of 4, 24, 48, 72, 96, 120, 144 and 168 h post-injection. The optimal imaging time point for the orthotopic mice was determined based on the subcutaneous tumor-bearing mice. Both preclinical imaging with the Pearl Trilogy Small Animal Imaging System (LI-COR, 800 nm channel; excitation 785 nm, emission filter 820 nm) and clinical imaging using the Artemis NIR Imaging system (Quest Medical Imaging b.v., Wieringerwerf, The Netherlands; excitation 780 nm, emission filter 805 nm) were employed for all imaging procedures. During imaging, mice were maintained under 2–4% isoflurane anesthesia. After the final measurement, mice were euthanized, and tumors and/or organs were excised for imaging using the Pearl imaging system. Tumor and background MFIs were computed by drawing regions of interest (ROIs) over the tumor area and adjacent normal tissue. These values were then included as individual data points for analysis. Image analysis was conducted using Image Studio (version 5.2, LI-COR) for Pearl images and Spectrum Capture Suite (Quest Medical Imaging b.v.) along with ImageJ (version 1.50, National Institutes of Health, Bethesda, MD, USA) for Quest images. Tumor-to-background ratios (TBRs) were determined by the formula: TBR = MFItumor/MFIbackground. For biodistribution analysis, organ MFIs were calculated by drawing a region of interest (ROIs) over the resected organ areas.
In vivo PA imaging
PA imaging was conducted 96 h following the injection using the Vevo 3100 Imaging System (FUJIFILM VisualSonics, Canada), following previously established protocols [33]. The imaging setup consisted of the Vevo LAZR-X cart, Vevo LAZRTight Enclosure, and Vevo Imaging Station. Mice were anesthetized and positioned on a prewarmed imaging table. The MX550D transducer from FUJIFILM VisualSonics was employed for both US and PA imaging (frequency range: 25–55 MHz; axial resolution: 40 μm; excitation at 780 nm). Subsequent image analysis was performed using Vevo LAB software (version: 5.5.0, FUJIFILM, VisualSonics).
Histological analysis of resected tumor tissue
Resected tumors were fixed overnight in 4% paraformaldehyde and subsequently dehydrated using ethanol. Afterwards, the tumor tissues were embedded in paraffin. Four-µm-thick formalin-fixed paraffin-embedded tissue sections were deparaffinized in xylene for 15 min, after which fluorescence imaging was performed using the Odyssey CLx Infrared Imaging System on the 800 nm channel. For immunofluorescence, slides were stained with ProLong Gold containing DAPI. Imaging of the slides was performed using the DAPI channel and the Cy7 channel of the Axio Scan Z1, as described before.
Statistical analysis
GraphPad Prism (version 9.3.1, GraphPad Software Inc., La Jolla, CA, USA) was used for statistical computations and the creation of graphs. IBM SPSS statistics version 29 (IBM Corporation, Armonk, NY, USA) was employed for all statistical analyses of patient characteristics, using a Chi-square test for categorical data, an unpaired t-test for normally distributed data, or the Mann–Whitney U test for nonparametric data. Differences between median TIS values on tumor and healthy tissue were compared using a Mann-Whitney U test. Differences between TBRs at different time points were compared using two-way ANOVA with Šídák correction for multiple comparisons. Differences with a P-value smaller than 0.05 were regarded as significant (ns: not significant. *P: ≤ 0.05, **P: ≤ 0.01, ***: P ≤ 0.001, ****: P ≤ 0.0001).
Results
Lea/c/x and sdi-Lea expression in malignant and healthy gastric and colorectal tissue
Tissue specimens of fifty-two gastric cancer and thirty-six colorectal cancer patients were obtained. Patient characteristics are summarized in Tables 1 and 2 of the Electronic Supplementary Materials (ESM). In total, tumor/healthy surrounding tissue specimens of 35/31 colorectal cancer and 52/43 gastric cancer patients were included for analysis. To evaluate the suitability of CH88.2 and CH129 as targeting moieties for NIRF imaging, the cohort of malignant and healthy tissue specimens was stained for Lea/c/x and sdi-Lea expression (Fig. 1) using their (mouse anti-human) counterparts FG88.2 and FG129, respectively. Positive Lea/c/x expression in gastric and colorectal cancer tissue specimens was observed in 81% and 83% of cases, respectively (Table 1). Positive sdi-Lea expression was found in 61% and 66% of gastric and colorectal cancer cases, respectively. Staining was mostly located on the cell membrane and showed a relatively homogenous staining distribution throughout the tumor, while some tissue slides showed a more heterogenous staining distribution. Categorized staining distributions on both malignant and healthy gastric and colorectal tissue are also shown in Table 1. Positive staining on tumor tissue specimens was predominantly strong for both biomarkers. While sdi-Lea expression on healthy surrounding tissue specimens was– if present– mostly weak, Lea/c/x expression on healthy surrounding tissue specimens was stronger, especially on healthy surrounding colorectal tissue. As shown in Table 2; Fig. 2, median biomarker staining, as expressed by the TIS, was significantly higher in tumor tissue compared to healthy surrounding tissue for all tumor types and both biomarkers. One exception was Lea/c/x expression in colorectal tissue specimens, which was significantly higher in healthy surrounding colorectal tissue compared to tumor tissue (9.0 vs. 6.0, P = 0.0021).
Fig. 1.
Representative images of HE and immunohistochemical expression of Lea/c/x and sdi-Lea on malignant and healthy surrounding gastric and colorectal tissue using mAbs FG88.2 and FG129, respectively. Scale bars represent 200 µM. Overview images and inserts are taken at 5× and 20× magnification, respectively. HE: hematoxylin-eosin
Table 1.
Distribution of Lea/c/x and sdi-Lea expression levels on gastric and colorectal cancer tissue as well as healthy surrounding tissue, as expressed by the TIS values categorized into negative (TIS = 0), weak (TIS = 1, 2, 3, 4) moderate (TIS = 6, 8) or strong expression (TIS = 9, 12)
| n = | Biomarker | Negative n (%) |
Weak n (%) |
Moderate n (%) |
Strong n (%) |
|||
|---|---|---|---|---|---|---|---|---|
| Gastric | Tumor | 52 | Lea/c/x | 10 (19) | 9 (17) | 5 (10) | 28 (54) | |
| 51 | sdi-Lea | 20 (39) | 6 (12) | 11 (22) | 14 (27) | |||
| Healthy | 43 | Lea/c/x | 26 (61) | 4 (9) | 3 (7) | 10 (23) | ||
| 41 | sdi-Lea | 33 (81) | 7 (17) | 0 (0) | 1 (2) | |||
| Colorectal | Tumor | 35 | Lea/c/x | 6 (17) | 8 (23) | 8 (23) | 13 (37) | |
| 35 | sdi-Lea | 12 (34) | 15 (43) | 5 (14) | 3 (9) | |||
| Healthy | 31 | Lea/c/x | 3 (10) | 0 (0) | 8 (26) | 20 (65) | ||
| 30 | sdi-Lea | 29 (97) | 1 (3) | 0 (0) | 0 (0) | |||
Table 2.
Comparison of quantified expression of Lea/c/x and sdi-Lea on gastric and colorectal cancer tissue vs. healthy surrounding tissue as expressed by the median TIS values, as well as their corresponding P-values. IQR: interquartile range
| Lea/c/x | sdi-Lea | ||||||
|---|---|---|---|---|---|---|---|
| Tumor type | Tissue | n = | Median (IQR) | P-value | n = | Median (IQR) | P-value |
| Gastric | Tumor | 52 | 9.0 (9.0) | < 0.0001 | 51 | 4.0 (9.0) | < 0.0001 |
| Healthy | 43 | 0.0 (8.0) | 41 | 0.0 (0.0) | |||
| Colorectal | Tumor | 35 | 6.0 (6.0) | 0.0021 | 35 | 3.0 (3.0) | < 0.0001 |
| Healthy | 31 | 9.0 (4.0) | 30 | 0.0 (0.0) |
Fig. 2.
Box plots representing TIS values of Lea/c/x and sdi-Lea expression on gastric and colorectal cancer as well as healthy surrounding tissue. Horizontal lines represent the median TIS, boxes represent interquartile range and brackets represent total TIS range. ns: not significant, TIS: tumor intensity score. *: P ≤ 0.05, **: P ≤ 0.01, ***: P ≤ 0.001, ****: P ≤ 0.0001
In vitro binding of CH88.2-800CW and CH129-800CW
CH88.2 and CH129 were successfully conjugated to IRDye 800CW (see Fig. 1 of the ESM). CH88.2-800CW and CH129-800CW binding was evaluated on a set of colon and pancreatic cancer cell lines using cell-based plate assays, considering their antigens’ presence on these human cancers. These tumor types were selected based on the abundant Lea/c/x and sdi-Lea expression in human tissue, allowing assessment of tracer binding across several relevant gastrointestinal malignancies. As shown in Fig. 3a-b, a concentration-dependent MFI increase was observed for both CH88.2-800CW and CH129-800CW on human colon adenocarcinoma cell line HT-29, as well as human pancreatic adenocarcinoma cell line BxPC-3, which was not observed for the remaining tumor cell lines. Comparison of absolute MFIs for CH88.2-800CW showed higher MFIs on HT-29 compared to BxPC-3, while for CH129-800CW MFIs were higher on BxPC-3 compared to HT-29 cells. HT-29 and BxPC-3 were selected as suitable cell lines for further in vivo evaluation with varying CH88.2 and CH129 binding levels. To confirm binding (localization) of CH88.2-800CW and CH129-800CW, immunofluorescence using cell-based chamber slides was performed which showed presence of both tracers on the membrane of HT-29 and BxPC-3 cells (Fig. 3c). In contrast, no binding of CH88.2-800CW and CH129-800CW was observed to COLO-320 and PANC-1, which was in line with the cell-based plate assay experiment that showed no MFI increase for these cell lines. Lastly, binding of negative control tracer rituximab-800CW to BxPC-3, HT-29, COLO-320 and PANC-1 cell lines was evaluated and was not observed, thereby establishing rituximab-800CW as a suitable negative control tracer for in vivo experiments.
Fig. 3.
In vitro binding of CH88.2-800CW and CH129-800CW to colon and pancreatic cancer cell lines. (a) Binding of CH88.2-800CW and CH129-800CW to colon cancer cell lines HT-29 (red), COLO-320 (blue), COLO-205 (green) and DLD-1 (yellow) at increasing concentrations using cell-based plate assays. (b) Binding of CH88.2-800CW and CH129-800CW to pancreatic cancer cell lines BxPC-3 (red), PANC-1 (blue) and MIA PaCa-2 (green) at increasing concentrations using cell-based plate assays. (c) Immunofluorescence analysis of CH88.2-800CW, CH129-800CW and rituximab-800CW binding to BxPC-3, HT-29, COLO-320 and PANC-1 using cell-based chamber slides. The 800CW signal is displayed in red and represents tracer localization. Nuclei are stained using DAPI and are displayed in blue. All experiments were performed in triplicate. a.u.: arbitrary units, MFI: mean fluorescence intensity
In vivo binding specificity of CH88.2-800CW and CH129-800CW
To evaluate binding specificity and establish the optimal imaging time point of CH88.2-800CW and CH129-800CW, mice were intravenously injected with 1 nmol of tracer and imaged for 168 h. For the subcutaneous HT-29_luc2 model, TBRs of > 2.0 were observed from 24 h post-injection onward for both CH88.2-800CW and CH129-800CW, which continued to increase until 168 h post-injection (Fig. 4a), albeit at the cost of lower tumor MFIs (see Fig. 2 of the ESM). From 48 h to 72 h onward, significantly higher TBRs were observed for CH88.2-800CW and CH129-800CW, respectively, compared to rituximab-800CW, indicating specificity of both glycan-binding tracers.
Fig. 4.
In vivo evaluation of CH88.2-800CW and CH129-800CW using subcutaneous HT-29_luc2 and BxPC-3_luc2 tumor-bearing mice. (a) Mean TBRs as a function of time after injection of 1 nmol CH88.2-800CW, CH129-800CW or rituximab-800CW to subcutaneous HT-29_luc2 tumor-bearing mice (n = 3/group). (b) Mean TBRs as a function of time post-injection of 1 nmol CH88.2-800CW, CH129-800CW or rituximab-800CW to subcutaneous BxPC-3_luc2 tumor-bearing mice (n = 3/group). Means are represented by the horizontal line, while error bars represent standard deviations (c) NIRF heatmap-color merge images of subcutaneous HT-29_luc2 and BxPC-3_luc2 tumor-bearing mice taken at 96 h post-injection of 1 nmol CH88.2-800CW, CH129-800CW or rituximab-800CW (n = 3/group). Images were captured using the preclinical Pearl NIRF imager and each mouse bears four tumors. MFI: mean fluorescence intensity, ns: not significant, p.i.: post-injection, s.c.: subcutaneous, TBR: tumor-to-background ratio **P < 0.01, ***P < 0.001, ****P < 0.0001
These in vivo results where verified in a BxPC-3_luc2 model, in which slightly lower TBRs were observed at all time points. From 72 to 48 h post-injection onward, respectively, TBRs for CH88.2-800CW and CH129-800CW, were significantly higher than those of rituximab-800CW, again indicating target specificity of CH88.2-800CW and CH129-800CW (Fig. 4b).
Considering the presence of a sufficiently high TBR as well as tumor MFI to allow clear tumor visualization, 96 h post-injection was established as the optimal imaging time point for both tracers. At this imaging time point, mean TBRs of 5.3 ± 0.8 and 3.5 ± 0.8 were observed for the subcutaneous HT-29_luc2 model using CH88.2-800CW and CH129-800CW, respectively, both allowing clear tumor visualization and delineation (CH88.2-800CW vs. rituximab-800CW: 95% CI 1.9; 5.2; P < 0.0001; CH129-800CW vs. rituximab-800CW: 95% CI 0.4; 3.2; P = 0.005) (Fig. 4a-c). For the subcutaneous BxPC-3 model, mean TBRs of 2.2 ± 0.3 and 2.3 ± 0.3 were observed for CH88.2-800CW and CH129-800CW, respectively, which also allowed clear tumor visualization (CH88.2-800CW vs. rituximab-800CW: 95% CI 0.2; 0.8; P = 0.0005; CH129-800CW vs. rituximab-800CW: 95% CI 0.4; 0.9; P < 0.0001) (Fig. 4a-c).
In vivo NIRF imaging potential of CH88.2-800CW and CH129-800CW
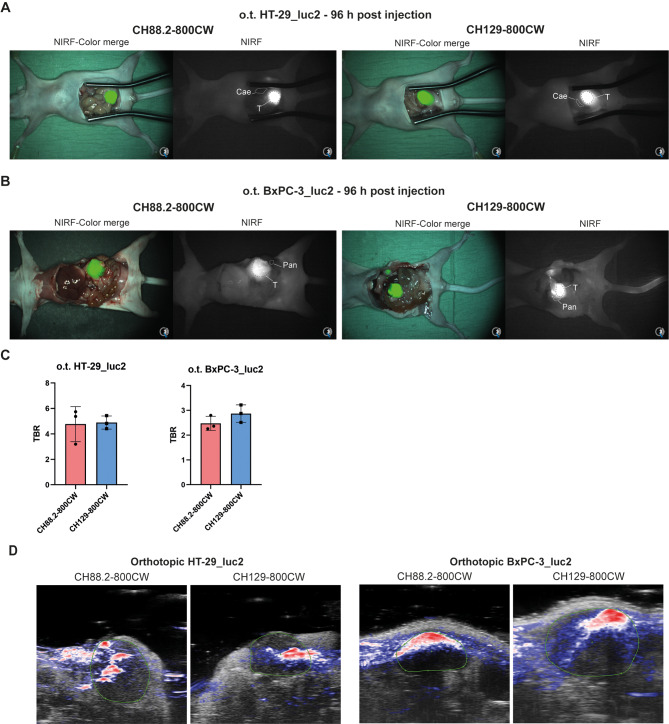
The NIRF imaging potential of CH88.2-800CW and CH129-800CW was evaluated in a clinically more relevant setting using orthotopic colon and pancreatic models. At 96 h post-injection of 1 nmol of each tracer, orthotopic HT-29_luc2 and BxPC-3_luc2 could be clearly delineated with high contrast using both tracers and the clinical Artemis NIRF imager (Fig. 5a-b). For the HT-29_luc2 model, mean TBRs of 4.8 ± 1.4 and 4.9 ± 0.5 were observed for CH88.2-800CW and CH129-800CW, respectively. For the BxPC-3_luc2, CH88.2-800CW and CH129-800CW TBRs were 2.5 ± 0.3 and 2.9 ± 0.4, respectively (Fig. 5c). This establishes both tracers as suitable for visualization of both higher and lower Lea/c/x and sdi-Lea-expressing tumors.
Fig. 5.
In vivo evaluation of CH88.2-800CW and CH129-800CW in orthotopic HT-29_luc2 and BxPC-3_luc2 tumor-bearing mice. (a) NIRF heatmap-color merge images of orthotopic HT-29_luc2 tumor-bearing mice taken at 96 h post-injection of 1 nmol CH88.2-800CW and CH129-800CW (n = 3/group). (b) NIRF heatmap-color merge images of orthotopic BxPC-3_luc2 tumor-bearing mice taken at 96 h post-injection of 1 nmol CH88.2-800CW and CH129-800CW (n = 3/group). Images were captured using the clinical Artemis NIRF imager using an exposure time of 150 ms, allowing real-time imaging. “T” indicates the tumor location, while “Cae” indicates healthy cecal tissue, and “Pan” indicates healthy pancreatic tissue. (c) Mean TBRs of orthotopic HT-29_luc2 and BxPC-3_luc2 tumors after injection of 1 nmol of CH88.2-800CW or CH129-800CW (n = 3/group). Means are represented by the horizontal line, while error bars represent standard deviations. (d) Representative photoacoustic and ultrasound overlay of orthotopic HT-29_luc2 and BxPC-3_luc2 tumors at 96 h post-injection of 1 nmol of CH88.2-800CW or CH129-800CW. Macroscopically identified tumors are delineated using the green line. Images were captured using a penetration depth of approximately 1.5 cm. NIRF: near-infrared fluorescence, o.t.: orthotopic, TBR: tumor-to-background ratio
In vivo PA imaging potential of CH88.2-800CW and CH129-800CW
The bimodal NIRF/PA imaging potential of CH88.2-800CW and CH129-800CW was evaluated in orthotopic HT-29_luc2 and BxPC-3_luc2 tumor-bearing mice at 96 h post-injection of 1 nmol of CH88.2-800CW or CH129-800CW. As shown in Fig. 5d, strong PA signal is observed within all tumor ultrasound ROIs for both CH88.2-800CW and CH129-800CW and both tumor types while PA signal in adjacent tissue is lower, thus allowing tumor-specific PA imaging.
Biodistribution and histological analysis
Following NIRF/PA imaging, biodistribution of CH88.2-800CW and CH129-800CW was evaluated by resecting tumors and organs at 96 h post-injection followed by NIRF imaging. NIRF images of resected tumors and organs for both tracers and tumor types are shown in Fig. 6a. Macroscopic fluorescence allowed clear HT-29_luc2 and BxPC-3_luc2 identification using both tracers. For both CH88.2-800CW and CH129-800CW, tumor MFIs were higher compared to fluorescence signal in all remaining organs, including the liver and kidneys (Fig. 6a-b).
Fig. 6.
Biodistribution of CH88.2-800CW and CH129-800CW. (a) Macroscopic fluorescence images showing biodistribution in resected orthotopic HT-29_luc2 and BxPC-3_luc2 tumors, and healthy organs at 96 h post-injection of 1 nmol of CH88.2-800CW and CH129-800CW. “Lu” lung, “Ht” heart, “Pa” pancreas, “Sp” spleen, “St” stomach, “Int” small intestine, “Ce” cecum, “Re” rectum, “Mu” muscle, “Br” brain, “Sk” skin, “Li” liver, “Ki” kidneys, “Tu” tumor. Images were captured using the preclinical Pearl NIRF imager. (b) MFIs in resected orthotopic HT-29_luc2 and BxPC-3_luc2 tumors, and healthy organs at 96 h post-injection of 1 nmol of CH88.2-800CW and CH129-800CW along with their standard deviations. a.u.: arbitrary units, MFI: mean fluorescence intensity, o.t.: orthotopic
Histological analysis showed largely overlapping fluorescence with tumor tissue, as well as cytokeratin and FG88.2 or FG129 staining, thereby confirming binding specificity and complete tumor penetration after injection of CH88.2-800CW and CH129-800CW (Fig. 7a). While HT-29_luc2 tumors showed homogenous fluorescence for both tracers, CH88.2-800CW and CH129-800CW fluorescence was more heterogenous in BxPC3_luc2 tumors. As shown in Fig. 7b, immunofluorescence of resected HT-29_luc2 and BxPC3-luc2 tumors showed membranous localization of CH88.2-800CW and CH129-800CW, thereby confirming specific binding of both NIRF tracers to tumor cells.
Fig. 7.
Histological analysis of CH88.2-800CW and CH129-800CW. (a) HE images, NIRF and merged HE-NIRF images, as well as cytokeratin and Lea/c/x or sdi-Lea expression on resected orthotopic HT-29_luc2 or BxPC-3_luc2 tumors. Images are taken at 20× magnification and scale bars represent 50 µM. Tumors are delineated using dashed black lines. (b) Microscopic fluorescence images of resected HT-29_luc2 and BxPC-3_luc2 96 h after injection 1 nmol of CH88.2-800CW or CH129-800CW. Nuclei are stained using DAPI and are displayed in blue. The 800CW signal is displayed in red and represents tracer localization and shows a membranous staining pattern. Scale bars represent 25 µM. HE: hematoxylin-eosin
Discussion
This study showed that Lea/c/x and sdi-Lea-binding tracers CH88.2-800CW and CH129-800CW allow high-contrast visualization of tumors using bimodal NIRF/PA imaging at 96 h post-injection using the clinical Artemis NIRF imager, with low fluorescence signal in healthy surrounding organs. Considering the high and mostly tumor-specific expression of Lea/c/x and sdi-Lea across gastric, colorectal and pancreatic cancer, both tracers could be employed for real-time intraoperative imaging for the majority of gastrointestinal cancer patients [7, 12, 13].
This study built upon the previously published proof-of-concept evaluation of Lea/c/x-specific CH88.2-800CW for NIRF imaging and additionally described the potential of sdi-Lea-specific CH129-800CW, as well as provided an extended immunohistochemical evaluation of their target’s expression [6]. Lea/c/x was abundantly expressed in gastric and colorectal cancer tissue, with (mostly) weak expression in healthy surrounding tissue. Noteworthy, as Lea/c/x expression in healthy surrounding colorectal epithelia was higher compared to its malignant counterparts, CH88.2-800CW may be a less suitable tracer for NIRF/PA imaging of colorectal cancer. sdi-Lea, however, showed slightly lower expression in colorectal and gastric cancer tissues, but, more importantly, substantially lower expression in healthy surrounding tissue compared to Lea/c/x, thus classifying this target as more tumor-specific. Therefore, CH129 may provide higher NIRF imaging contrast in vivo, although its target sdi-Lea is expressed in fewer patients compared to CH88.2. These immunohistochemical data are consistent with previous immunohistochemical analyses of Lea/c/x and sdi-Lea expression in tumor and healthy surrounding tissue specimens [12, 13]. Although we focused on gastrointestinal cancers, the findings of this study might be extrapolated to other tumor types that expresses Lea/c/x and sdi-Lea glycan epitopes. Notably, the presence of both targets on roughly 20–30% of non-small cell lung cancer and ovarian cancer with limited staining on normal surrounding tissue, has been reported, thereby extending the potential application of both tracers beyond gastrointestinal cancers [12, 13].
The in vivo findings of this study were also consistent with those obtained in the previously published CH88-2-800CW pilot, in which we solely employed subcutaneous HT-29 and BxPC-3 tumor-bearing mice [6]. Compared to the BxPC-3 model, higher MFIs were observed at most time points for both tracers using the HT-29 model, consequently leading to higher TBRs, which was potentially caused by varying CH88.2 and CH129 binding to these cell lines. Nevertheless, both tumor models could be excellently delineated using CH88.2-800CW in both the previous and current study, suggesting the suitability of both tracers for imaging of tumors that express Lea/c/x and sdi-Lea to a lesser extent. Moreover, our orthotopic models allowed biodistribution analysis at the optimal imaging time point of 96 h post-injection. The observation that tumor fluorescence exceeded liver fluorescence at this time point is encouraging, as sufficient tumor-to-liver contrast is pivotal for this; a common metastatic site for gastrointestinal cancers [21]. Nevertheless, deeper-located hepatic metastases could be missed using NIRF imaging alone, considering the limited penetration depth of NIR light (~ 7 mm) [14].
As a solution, intraoperative tumor imaging may be enhanced by supplementing NIRF imaging with PA imaging, theoretically allowing detection of tumor tissue located beyond NIRF imaging’s penetration depth. Practically, a tumor may be approached using PA imaging and, once reached through PA-guided dissection, high-resolution NIRF imaging can be employed to guide radical tumor resection by overlaying the surgeon’s view with a real-time NIRF image. Bimodal NIRF/PA imaging using IRDye 800CW as a photosensitizer has been successfully demonstrated in previous research [22, 23]. The clinical application of NIRF/PA imaging was first described by Tummers et al. for pancreatic cancer using cetuximab-800CW, which provided a nearly 4-fold higher mean PA signal in tumor lesions ex vivo, compared to surrounding healthy pancreatic tissue [24].
Although glycan targeting is still in its infancy, targeting TACAs for imaging may offer advantages over ‘conventional’ protein targeting methods [5]. Apart from their low abundance in healthy tissue and dense expression in cancer, glycans are expressed at the outmost layer of the cell membrane, making them directly accessible by administered targeting moieties. Also, TACAs are present on multiple proteins simultaneously, enabling indirect targeting of multiple proteins through a single anti-glycan tracer administration. Lewis glycan-targeted molecular imaging has particularly focused on the application of sLea (CA19-9)-targeted agents in pancreatic cancer [5]. sLea is overexpressed in > 90% of pancreatic cancers and is commonly used as a serum biomarker for follow-up monitoring in this tumor type [25]. Lohrmann et al. performed a phase 1 PET imaging trial in pancreatic cancer using MVT-2163, which is comprised of the anti-sLea mAb HuMab-5B1 conjugated to 89Zr [26]. The tracer was well tolerated, causing mild to moderate side effect on the day of administration and provided high-contrast visualization of primary PDAC as well as metastases. Of note, additional sub-centimeter lesions near common metastatic sites that were invisible on conventional imaging were identified. However, the elevation of sLea in normal pancreatic tissue, chronic pancreatitis, cholangitis, obstructive jaundice and other benign conditions, reduces the potential of sLea-targeted tracers. Therefore, targeting more tumor-specific (glycan-based) targets for imaging of pancreatic cancer, such as Lea/c/x and sdi-Lea, may provide superior accuracy.
This study has some limitations. Firstly, the relatively small cohort size of the immunohistochemical evaluation of Lea/c/x and sdi-Lea expression hindered comprehensive subgroup analyses. Therefore, we could, for instance, not quantify the effect of neoadjuvant therapy (NAT) on Lea/c/x and sdi-Lea expression, which is known to affect biomarker expression in tumors [27–29]. Nevertheless, as a substantial portion of the included gastric and colorectal cancer patients did receive NAT and strong expression was maintained throughout the cohort, we anticipate the effect of NAT on biomarker expression to be limited. This is corroborated by previous research that showed no effect of neoadjuvant therapy on Lea/c/x and sdi-Lea expression in PDAC [7, 8].
Secondly, the in vivo models used in this study provide an estimation of clinical practice. As mice do not naturally express Lewis glycans due to lack of fucosyltransferase-3, the TBRs observed in this study could have been overestimated [30]. However, the in vivo findings should be considered alongside our immunohistochemical analysis of Lea/c/x and sdi-Lea expression in human tumors and healthy surrounding tissue specimens, which demonstrated sufficient tumor-specificity for most target/tumor type combinations. This suggests that sufficient imaging contrast could theoretically be achieved intraoperatively. Moreover, glycans such as sLea are known to be shed into the circulation, where they may be bound by a glycan-targeting tracer, thereby diverting away tracer from the tumor and reducing tumor-to-background contrast [31]. However, as extent of glycan shedding and its effect on tracer distribution was not investigated in this study, its influence on the in vivo imaging results could not be assessed. Nevertheless, the high TBRs observed in this study suggest that any such effect– if present– did not drastically impede tumor imaging under our experimental conditions. Lastly, the tumor/stromal composition as well as its heterogeneity is not properly represented in our in vivo tumor models. Given the inverse correlation between the amount of stromal tissue and tumor penetration of tracers, the increased amount of stroma in human tumors may hamper tumor penetration in clinical practice. Although patient-derived xenografts, -organoids or complex co-culture models could better approach the human tumor composition, these still provide an estimation [32, 33]. Therefore, clinical evaluation of the CH88.2-800CW and CH129-800CW in gastrointestinal cancer patients is warranted to establish their suitability for bimodal NIRF/PA imaging. Considering the established clinical use of IRDye 800CW, rapid clinical translation is feasible.
Conclusions
Our findings showed that bimodal NIRF/PA imaging using CH88.2-800CW and CH129-800CW allows real-time, high-contrast visualization of tumors at 96 h post-injection. Considering the strong and tumor-specific expression of Lea/c/x and sdi-Lea on gastric, colorectal, and pancreatic cancer, both tracers may be broadly applied for gastrointestinal cancers. This preclinical evaluation warrants further evaluation of both agents in a clinical setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Cornelis Sier for his support and involvement throughout the study.
Author contributions
Material preparation, data collection and analysis were performed RDH, VQS, MvD, VMB, TS, DK and ASLPC. The first draft of the manuscript was written by RDH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No external funding was obtained for this study.
Data availability
The data presented in this study are available upon reasonable request from the corresponding author.
Declarations
Ethical approval
The research protocol was approved by the Gastroenterology Biobank Review Committee and the local Medical Ethical Review Committee. The study strictly adhered to the Dutch code of conduct for responsible use of human tissue in medical research. All tissue specimens and associated clinicopathological data were utilized in an anonymized manner and in accordance with the principles outlined in the Declaration of Helsinki (1964). The local animal welfare body of the LUMC reviewed and approved all animal studies. Animals were humanely cared for in accordance with the Code of Practice Animal Experiments in Cancer Research and guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
Consent to participate
The need for informed consent was waived by the local Medical Ethical Review Committee.
Consent for publication
Not applicable.
Conflict of interest
Lindy G. Durrant is CEO and CSO of Scancell Ltd. and has ownership interest in Scancell Ltd. Mireille Vankemmelbeke is employed at Scancell Ltd. All remaining authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
9/2/2025
A Correction to this paper has been published: 10.1186/s13550-025-01308-5
References
- 1.Khan MA, Hakeem AR, Scott N, Saunders RN. Significance of R1 resection margin in colon cancer resections in the modern era. Colorectal Dis. 2015;17(11):943–53. 10.1111/codi.12960. [DOI] [PubMed] [Google Scholar]
- 2.Vahrmeijer AL, Frangioni JV. Seeing the invisible during surgery. Br J Surg. 2011;98(6):749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mieog JSD, Achterberg FB, Zlitni A, Hutteman M, Burggraaf J, Swijnenburg R-J, et al. Fundamentals and developments in fluorescence-guided cancer surgery. Nat Reviews Clin Oncol. 2021. 10.1038/s41571-021-00548-3. [DOI] [PubMed] [Google Scholar]
- 4.Hernot S, van Manen L, Debie P, Mieog JSD, Vahrmeijer AL. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019;20(7):e354–67. 10.1016/S1470-2045(19)30317-1. [DOI] [PubMed] [Google Scholar]
- 5.Houvast RD, Vankemmelbeke M, Durrant LG, Wuhrer M, Baart VM, Kuppen PJK, et al. Targeting glycans and heavily glycosylated proteins for tumor imaging. Cancers (Basel). 2020;12(12). 10.3390/cancers12123870. [DOI] [PMC free article] [PubMed]
- 6.Houvast RD, Baart VM, Bhairosingh SS, Cordfunke RA, Chua JX, Vankemmelbeke M, et al. Glycan-Based Near-infrared fluorescent (NIRF) imaging of Gastrointestinal tumors: a preclinical Proof-of-Concept in vivo study. Mol Imaging Biol. 2020. 10.1007/s11307-020-01522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houvast RD, Thijse K, Groen JV, Chua J, Vankemmelbeke M, Durrant LG, et al. An immunohistochemical evaluation of Tumor-Associated glycans and mucins as targets for molecular imaging of pancreatic ductal adenocarcinoma. Cancers (Basel). 2021;13(22). 10.3390/cancers13225777. [DOI] [PMC free article] [PubMed]
- 8.Houvast RD, van Duijvenvoorde M, Chua J, Vankemmelbeke M, Durrant LG, Inderson A, et al. Prediction of biomarker expression on primary pancreatic ductal adenocarcinoma tissues using Fine-Needle biopsies: paving the way for a Patient-Tailored molecular imaging approach. Mol Diagn Ther. 2023;27(2):261–73. 10.1007/s40291-022-00635-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munkley J, Elliott DJ. Hallmarks of glycosylation in cancer. Oncotarget. 2016;7(23):35478–89. 10.18632/oncotarget.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuster MM, Esko JD. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer. 2005;5(7):526–42. 10.1038/nrc1649. [DOI] [PubMed] [Google Scholar]
- 11.Contessa JN, Bhojani MS, Freeze HH, Rehemtulla A, Lawrence TS. Inhibition of N-linked glycosylation disrupts receptor tyrosine kinase signaling in tumor cells. Cancer Res. 2008;68(10):3803–9. 10.1158/0008-5472.Can-07-6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua JX, Vankemmelbeke M, McIntosh RS, Clarke PA, Moss R, Parsons T, et al. Monoclonal antibodies targeting LecLex-Related glycans with potent antitumor activity. Clin Cancer Res. 2015;21(13):2963–74. 10.1158/1078-0432.CCR-14-3030. [DOI] [PubMed] [Google Scholar]
- 13.Tivadar ST, McIntosh RS, Chua JX, Moss R, Parsons T, Zaitoun AM, et al. Monoclonal antibody targeting Sialyl-di-Lewisa - Containing internalizing and non-Internalizing glycoproteins with Cancer immunotherapy development potential. Mol Cancer Ther. 2019. 10.1158/1535-7163.Mct-19-0221. [DOI] [PubMed] [Google Scholar]
- 14.Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJH, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Reviews Clin Oncol. 2013;10(9):507–18. 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attia ABE, Balasundaram G, Moothanchery M, Dinish US, Bi R, Ntziachristos V, et al. A review of clinical photoacoustic imaging: current and future trends. Photoacoustics. 2019;16:100144. 10.1016/j.pacs.2019.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao J, Chen J, Ma S, Liu Q, Huang L, Chen X, et al. Recent developments in multimodality fluorescence imaging probes. Acta Pharm Sin B. 2018;8(3):320–38. 10.1016/j.apsb.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vermeulen I, Isin EM, Barton P, Cillero-Pastor B, Heeren RMA. Multimodal molecular imaging in drug discovery and development. Drug Discov Today. 2022;27(8):2086–99. 10.1016/j.drudis.2022.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Mahler Convenor M, Berard M, Feinstein R, Gallagher A, Illgen-Wilcke B, Pritchett-Corning K, et al. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim. 2014;48(3):178–92. 10.1177/0023677213516312. [DOI] [PubMed] [Google Scholar]
- 19.Tseng W, Leong X, Engleman E. Orthotopic mouse model of colorectal cancer. J Vis Exp. 2007;10:484. 10.3791/484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno JA, Sanchez A, Hoffman RM, Nur S, Lambros MP. Fluorescent orthotopic mouse model of pancreatic Cancer. J Vis Exp. 2016;11510.3791/54337. [DOI] [PMC free article] [PubMed]
- 21.Horn SR, Stoltzfus KC, Lehrer EJ, Dawson LA, Tchelebi L, Gusani NJ, et al. Epidemiology of liver metastases. Cancer Epidemiol. 2020;67:101760. 10.1016/j.canep.2020.101760. [DOI] [PubMed] [Google Scholar]
- 22.Baart VM, van der Horst G, Deken MM, Bhairosingh SS, Schomann T, Sier VQ, et al. A multimodal molecular imaging approach targeting urokinase plasminogen activator receptor for the diagnosis, resection and surveillance of urothelial cell carcinoma. Eur J Cancer. 2021;146:11–20. 10.1016/j.ejca.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Houvast RD, Badr N, March T, de Muynck L, Sier VQ, Schomann T, et al. Preclinical evaluation of EpCAM-binding designed Ankyrin repeat proteins (DARPins) as targeting moieties for bimodal near-infrared fluorescence and photoacoustic imaging of cancer. Eur J Nucl Med Mol Imaging. 2023. 10.1007/s00259-023-06407-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tummers WS, Willmann JK, Bonsing BA, Vahrmeijer AL, Gambhir SS, Swijnenburg RJ. Advances in diagnostic and intraoperative molecular imaging of pancreatic Cancer. Pancreas. 2018;47(6):675–89. 10.1097/mpa.0000000000001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poruk KE, Gay DZ, Brown K, Mulvihill JD, Boucher KM, Scaife CL, et al. The clinical utility of CA 19– 9 in pancreatic adenocarcinoma: diagnostic and prognostic updates. Curr Mol Med. 2013;13(3):340–51. 10.2174/1566524011313030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohrmann C, O’Reilly EM, O’Donoghue JA, Pandit-Taskar N, Carrasquillo JA, Lyashchenko SK, et al. Retooling a Blood-Based biomarker: phase I assessment of the High-Affinity CA19-9 antibody HuMab-5B1 for Immuno-PET imaging of pancreatic Cancer. Clin Cancer Res. 2019;25(23):7014–23. 10.1158/1078-0432.Ccr-18-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan H, Wang R, Yu J. Predictive and prognostic value of Smac, VEGF and Ki67 expression in locally advanced rectal Cancer treated with neoadjuvant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;78(3):S197. [Google Scholar]
- 28.Boogerd LS, van der Valk MJ, Boonstra MC, Prevoo HA, Hilling DE, van de Velde CJ, et al. Biomarker expression in rectal cancer tissue before and after neoadjuvant therapy. Onco Targets Ther. 2018;11:1655–64. 10.2147/ott.S145473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohan SC, Walcott-Sapp S, Lee MK, Srour MK, Kim S, Amersi FF, et al. Alterations in breast Cancer biomarkers following neoadjuvant therapy. Ann Surg Oncol. 2021;28(11):5907–17. 10.1245/s10434-021-09814-1. [DOI] [PubMed] [Google Scholar]
- 30.Gersten KM, Natsuka S, Trinchera M, Petryniak B, Kelly RJ, Hiraiwa N, et al. Molecular cloning, expression, chromosomal assignment, and tissue-specific expression of a murine alpha-(1,3)-fucosyltransferase locus corresponding to the human ELAM-1 ligand Fucosyl transferase. J Biol Chem. 1995;270(42):25047–56. 10.1074/jbc.270.42.25047. [DOI] [PubMed] [Google Scholar]
- 31.Houghton JL, Abdel-Atti D, Scholz WW, Lewis JS. Preloading with unlabeled CA19.9 targeted human monoclonal antibody leads to improved PET imaging with 89Zr-5B1. Mol Pharm. 2017;14(3):908–15. 10.1021/acs.molpharmaceut.6b01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.1021/acs. molpharmaceut.6b01130. Epub 2017 Feb 21.
- 32.Harryvan TJ, Hawinkels L, Östman A, Ten Dijke P, Strell C, Hornsveld M. A novel pancreatic Cancer Mini-tumor model to study desmoplasia and myofibroblastic Cancer-Associated fibroblast differentiation. Gastro Hep Adv. 2022;1(4):678–81. 10.1016/j.gastha.2022.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hakuno SK, Michiels E, Kuhlemaijer EB, Rooman I, Hawinkels L, Slingerland M. Multicellular modelling of Difficult-to-Treat Gastrointestinal cancers: current possibilities and challenges. Int J Mol Sci. 2022;23(6). 10.3390/ijms23063147. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.