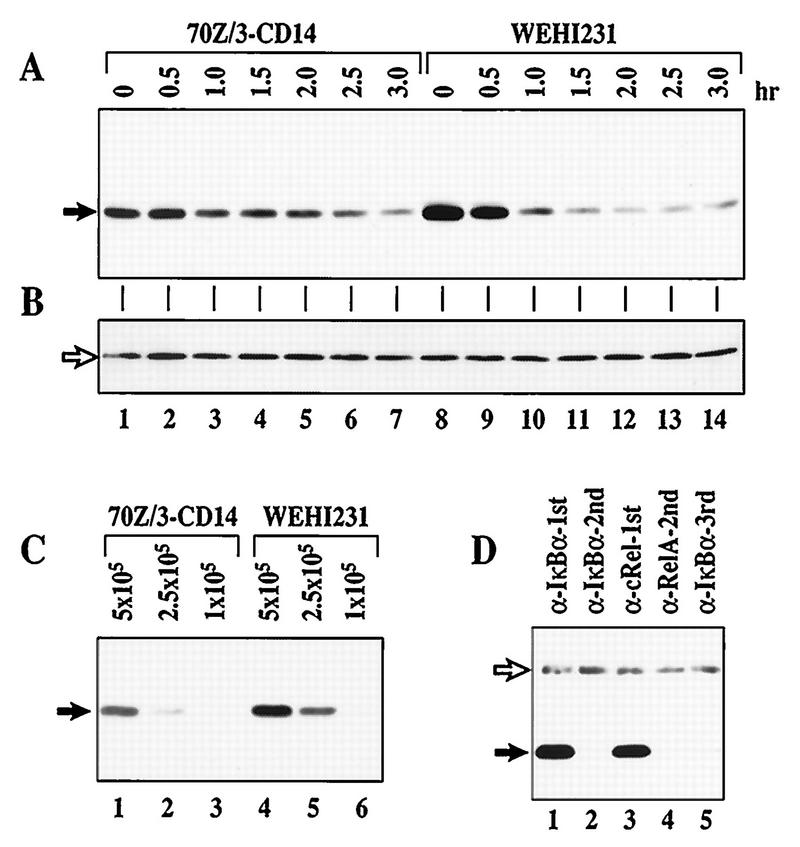
FIG. 1.
IκBα is associated with c-Rel and undergoes rapid proteolysis in WEHI231 cells. (A) Total IκBα degrades faster in WEHI231 cells than in 70Z/3-CD14 cells. The same number of 70Z/3-CD14 and WEHI231 cells (1.4 × 106) were incubated with cycloheximide (20 μg/ml) and terminated at the time points shown. Total cell pellets were dissolved in 2× Laemmli sample buffer and immediately boiled to preserve potentially modified IκBα forms. The samples were electrophoresed in SDS–12.5% polyacrylamide gels, transferred to a nylon membrane, and probed with IgG against IκBα protein. The protein bands (arrow) were visualized by ECL reaction. (B) Loading control for blot in panel A. The blot in panel A was reprobed with IgG against RelA and developed as described above; a nonspecific band (arrow) is shown. (C) Relative steady-state levels of IκBα in WEHI231 and 70Z/3-CD14 cells. Serial dilutions of 70Z/3-CD14 and WEHI231 cells (shown above each lane in cell number) were loaded. Positions of IκBα are shown on the left (arrow). (D) IκBα is complexed exclusively with c-Rel in WEHI231 cells. WEHI231 cells (106) were lysed in a hypotonic buffer in the presence of various protease inhibitors and phosphatase inhibitors as described in Materials and Methods, and the cytoplasmic fraction was split into two equal fractions. One fraction was immunoprecipitated with antibody against IκBα (lane 1), and the unprecipitated supernatant was reimmunoprecipitated to examine the efficiency of the first precipitation (lane 2). The other half of the original fraction was first immunoprecipitated with anti-c-Rel (lane 3). The unprecipitated proteins were then immunoprecipitated with anti-RelA (lane 4), and the same procedure was repeated for final IκBα precipitation (lane 5). The immunoprecipitates were electrophoresed in SDS–10% polyacrylamide gels, blotted, and probed with anti-IκBα antibody. The IκBα band (filled arrow) was visualized by ECL reaction using HRP-conjugated protein A to reduce reactivity with the rabbit Igμ heavy chains used for immunoprecipitation (open arrow).