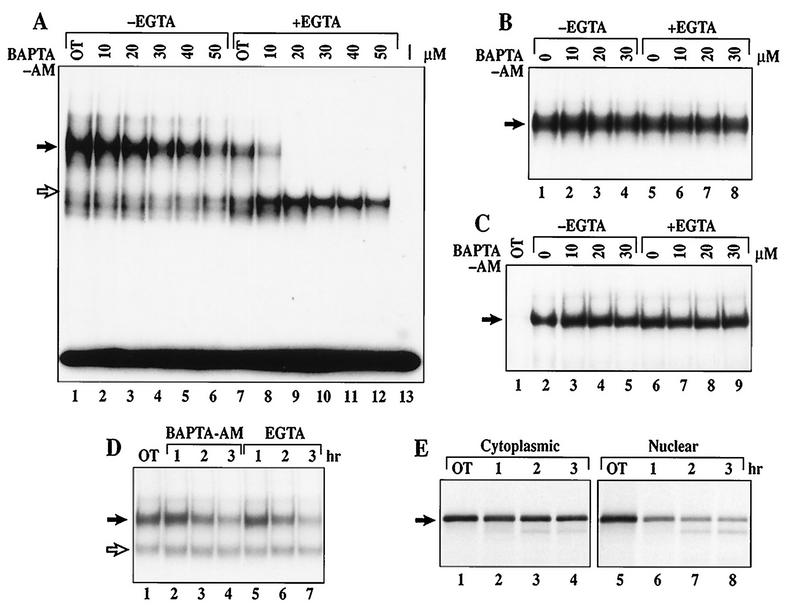
FIG. 7.
Calcium is essential for the maintenance of constitutive p50–c-Rel activity in WEHI231 cells. (A) EMSA of WEHI231 cells treated with BAPTA-AM with or without EGTA. WEHI231 cells were treated with various doses of BAPTA-AM without (lanes 2 to 6) or with (lanes 8 to 12) EGTA (2.5 mM). Lane 1, DMSO alone (0.2%); lane 7, DMSO plus EGTA; lane 13, without a nuclear extract. Position of the p50–c-Rel heterodimer is shown by the filled arrow, whereas a p50 homodimer is shown by an open arrow. (B) BAPTA-AM and EGTA do not directly block p50–c-Rel DNA binding activity. A nuclear extract prepared from untreated WEHI231 cells was incubated with doses of BAPTA-AM (lanes 2 to 4), EGTA (lane 5), or BAPTA-AM plus EGTA (lanes 6 to 8) for 40 min and analyzed by EMSA. An area of the gel with p50–c-Rel complex is shown (arrow). (C) BAPTA-AM and EGTA do not block LPS-induced p50–RelA binding activity in 70Z/3-CD14 cells. 70Z/3-CD14 cells were treated with doses of BAPTA-AM without (lanes 3 to 5) or with (lanes 7 to 9) EGTA or with EGTA alone (lane 6) and treated with LPS (1 μg/ml) for 15 min, and nuclear extracts were analyzed by EMSA. Lane 1, unstimulated cells; lane 2, DMSO- and LPS-treated cells. An area of the gel with p50-RelA complex is shown (arrow). (D) Time course of inhibition of p50–c-Rel binding in WEHI231 cells by BAPTA-AM and EGTA. WEHI231 cells were treated with either BAPTA-AM (30 μM; lanes 2 to 4) or EGTA (2.5 mM; lanes 5 to 7) for the indicated periods of time. Nuclear extracts were analyzed by EMSA as described above. Lane 1, untreated cells. The filled arrow points to p50–c-Rel, while the open arrow points to p50 homodimer. (E) Pulse-chase of cytoplasmic and nuclear c-Rel protein in WEHI231 cells. WEHI231 cells were pulse-labeled with [35S]Met-Cys for 3.5 h, washed with growth medium, and incubated in growth medium, and equal cell numbers were terminated at time points shown. The cells were then fractionated into cytoplasmic and nuclear pools, and each pool was immunoprecipitated with anti-c-Rel antibody. Cytoplasmic fractions used for immunoprecipitation were one-fourth the level of the nuclear fractions for each time point. The exposure time for nuclear and the cytoplasmic fractions was the same (3 days). Quantification by PhosphorImager demonstrated that the half-life of cytoplasmic c-Rel was >3 h, while that for nuclear c-Rel was 57 min.