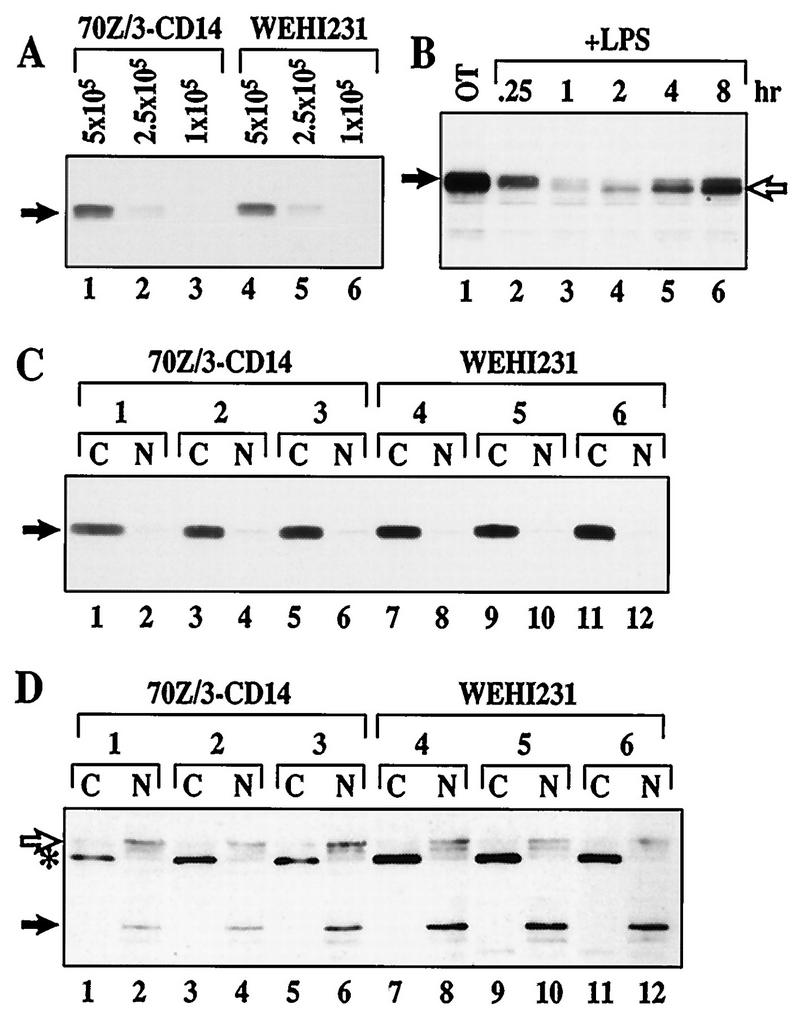
FIG. 9.
Level, migration pattern, and subcellular localization of IκBβ are the same in WEHI231 cells as in 70Z/3-CD14 cells. (A) Steady-state level of IκBβ in WEHI231 and 70Z/3-CD14 cells. The Western blot shown in Fig. 1C was also probed with anti-IκBβ antibody to examine the relative level of IκBβ protein (arrow). Samples were as in Fig. 1C. (B) Hypophosphorylated IκBβ produced following prolonged stimulation of 70Z/3-CD14 with LPS. 70Z/3-CD14 cells were treated with LPS (1 μg/ml) for up to 8 h. Equal fractions of cells were terminated at each time point and analyzed by Western blotting using anti-IκBβ antibody. The filled arrow shows basally phosphorylated IκBβ, while the open arrow points to newly synthesized hypophosphorylated IκBβ (lanes 4 to 6). (C) IκBβ is cytoplasmic in both WEHI231 and 70Z/3-CD14 cells. Three sets of 70Z/3-CD14 and WEHI231 cells were fractionated into cytoplasmic (C) and nuclear (N) fractions independently as described in Materials and Methods. The resulting fractions were analyzed by Western blotting using anti-IκBβ antibody. (D) Sp-1 and lamin B are nuclear. The blot in panel C was reprobed with antibodies against nuclear proteins Sp-1 (open arrow) and lamin B (closed arrow). The bands were visualized with HRP-conjugated anti-mouse antibody followed by ECL reaction. An asterisk points to an unknown protein which is exclusively localized in the cytoplasmic fraction.