Abstract
Artificial implants have consistently been recognized as the most effective clinical strategy for repairing bone fractures and defects, particularly in orthopedics and stomatology. Nowadays, the focus of bone repair has shifted from basic fixation and structural restoration to the reconstruction of multifunctional “live” tissue to mimic the natural bone microenvironment. However, developing the smart implants with ideal osteogenesis-related multi-functions remains challenging, as the effects of physicochemical properties of implant materials on intracellular signaling, stem cell niches, and tissue regeneration are not yet fully understood. Herein, we systematically explore recent advancements in innovative strategies for bone repair and regeneration, revealing the significance of the smart implants that closely mimic the natural structure and function of bone tissue. Adaptation to patient-oriented osteogenic microenvironments, dynamic osteoblastogenesis-osteoclastogenesis balance, antibacterial/bactericidal capacity, vascularization, and osteoimmunomodulatory capacity and their regulatory mechanisms achieved by biomaterials design and functional modifications are thoroughly summarized and analyzed. Notably, the popular research on multifunctional platforms with synergetic interactions between different functions and treatment of complex clinical issues, including the emerging neurogenic bone repair, is also significantly discussed for developing more intelligent implants. By summarizing recent research efforts, this review proposes the latest multifunctional strategies and synergistic mechanisms of smart bone implants, aiming to provide better bone defect repair applications that more closely mimic the natural bone tissue.
Keywords: Smart bone implants, Osteogenesis, Multifunctional strategy, Synergistic mechanism, Neurogenic bone repair
Graphical abstract
Highlights
-
•
Smart implants tend to more closely mimic and regenerate natural bone tissue.
-
•
Patient-oriented osteogenic microenvironments and bone metabolism are important.
-
•
Antibacterial capacity, vascularization, and osteoimmunomodulation are main osteogenesis-related functions.
-
•
Smart implants achieve synergetic interactions between different functions and complex clinical issues.
-
•
Herein, multifunctional strategies can promote more complex bone reconstruction.
1. Introduction
Bone injuries and defects caused by trauma, tumors, osteoporosis, or severe infection present significant challenges in clinical practice, particularly in orthopedics and stomatology, seriously impairing the quality of millions of individuals life [[1], [2], [3]]. Among the development of aging society, the orthopaedic disorders already occupy a significant proportion of the global burden of disease, notably in the low and middle-income countries, and its injury has reached 11.2 % of the total disability-adjusted life-years worldwide [4,5]. Hence, the demand for clinical therapy techniques and corresponding high-performance implants of the orthopaedic injury is becoming more urgent. Traditional approaches to bone repair, such as autografts and allografts, which have many limitations, including limited donor resource, risk of infection, and immunological rejection, prompting the development of artificial implants as more effective solutions [6,7]. Over the years, the focus of bone repair strategies has evolved from mere structural fixation and shape restoration to the reconstruction of living and multifunctional tissues that closely mimic the natural bone microenvironment [8].
During continual evolution, human skeleton with hundreds of bones endows the unique shape and macro/microstructures [9], which mainly provide the role of supporting human tissues and organs, shaping human body (mechanical function) [10]; producing blood cells (i.e., hematopoiesis) by bone marrow in cancellous part (synthetic function) [11]; releasing of neurogenic factor by the highly neurovascular coupling (secretory function) [12]; keeping the balance of mineral, fat and pH value [13], and detoxicating (metabolic function) [14]. To achieve the biological performance closer to natural bone such as sufficient bioactivity [15], biocompatibility [16], osteogenesis [17], osteoconduction [18], and osteoinduction [19], the concept of “smart bone implants” is gradually emerging. Unlike traditional implants that primarily provide mechanical support, the smart bone implants actively interact with the biological microenvironment, facilitating bone repair and regeneration to match the microstructure and above functions of natural bone tissue as much as possible [20]. By integrating advanced biomaterials, bioinspired structural designs, and functional adaptability, these implants dynamically regulate cellular responses, promote bone regeneration, and optimize microstructural and functional compatibility with natural bone tissue [21]. As shown in Fig. 1, an ideal smart bone implant should not only replicate the natural bone's microstructure but also integrate intelligent, multifunctional features that dynamically influence bone repair and regeneration. This requires a multifunctional approach, integrating features such as being endowed with interconnected porous structure, sufficient strength and hardness, anticipated osteogenesis, bacteriostasis and anti-infection, rational regulation of immune response, promoting internal neovascularization and blood circulation, regaining the awareness of pain and perception (nerve repair and regeneration), and tightly combining with bone tissue in vivo [[22], [23], [24], [25]]. Thus, the development of smart bone implants necessitates a comprehensive and synergistic design strategy that mimics the structural, mechanical, and biological complexity of natural bone while actively modulating the local microenvironment to achieve enhanced functional integration and long-term clinical efficacy.
Fig. 1.
Schematic representation of the functional design framework for smart bone implants integrating material properties, regulating cellular behaviours and synergistic functions, and fulfilling clinical requirements.
With the continuous clinical application of metal implants, the strong binding ability of implant to natural bone tissue (also osseointegration) is urgently demanded to overcome the clinical problems such as fractures, nonunion, implant loosening and failure, and periprosthetic infection [26,27]. The emergence of smart implants offers innovative solutions that integrate advanced materials science with biological mechanisms, enabling not only structural support but also active facilitation of the healing process by enhancing osteogenesis, preventing infections, promoting vascularization, modulating the immune response, and supporting neuromodulatory osteogenesis [12,[28], [29], [30]]. These functionalities are achieved through precise material designs, including bioactive coatings, surface nanostructures, and other surface modifications, which dynamically interact with the cellular microenvironment to enhance clinical therapeutic efficacy [31,32]. In recent years, researchers have been devoted to developing smart bone implants, and optimizing existing functions and fabrication approaches of the implants such as effective combination of the above functions, induction and modification of loaded bioactive complexes (e.g., exosome and functional protein). For example, the active regulation of macrophage polarization (M1 and M2 phenotype) is also increasingly studied through designing surface topography of bone implants and introducing some drugs, cytokines and active metal ions, verifying that the immunomodulatory effect of implant is also one of the important functions of osteointegration in bone defect repair [[33], [34], [35]]. However, the smart bone implants proposed inhere has not yet been systematically elaborated in bone tissue engineering. Simultaneously, Simultaneously, when facing complex clinical challenges such as septic prosthetic joint loosening, it is crucial to dynamically regulate biological processes-including osteogenesis, antibacterial properties, vascularization, immunomodulation, and neural regeneration-to restore osseointegration between the implant and natural bone tissue [26]. Therefore, achieving synergistic integration of multifunctional capabilities into implants and developing smart bone implants with functionalities comparable to natural bone tissue remains challenging. A deeper understanding of how the physiochemical properties of implant materials influence intracellular signaling pathways, stem cell niches, and bone regeneration processes is critical for the development of next-generation smart implants. Specifically, integrating dynamic and adaptive functionalities during the bone healing process, such as four-dimensional (4D)-printed implants that can change shape in response to physiological microenvironment, along with strategies that facilitate crosstalk between skeletal and other biological systems, making it more closely mimic the dynamic and hierarchical architecture of natural bone regeneration.
Herein, we systematically present the latest strategies and research perspectives on smart implants for bone tissue repair and regeneration, drawing upon hundreds of relevant publications from the past decade. We comprehensively analyze the patient-specific osteogenic microenvironments encountered in clinical bone repair and the regulatory mechanisms of osteogenesis-related signaling pathways facilitated by smart implants. Notably, the research strategies and synergetic mechanism of multifunctional platforms that closely mimic the performance of natural bone including the neural regeneration within bone tissue, are innovatively summarized and systematically discussed for developing more intelligent implants. This review offers the most recent insights into bone repair, spanning from biomedical materials to underlying biological mechanisms, aiming to provide both technical and theoretical support for the innovative design and clinical application of smart implants in bone repair and regeneration.
2. Osteogenic functions and mechanisms of smart bone implants
Osteogenesis is a vital and basical process in the regenerative healing of skeletal muscle systems, including instances such as fractures [36], total joint arthroplasty [37], and resection of bone tumors [38] during the postoperative recovery stage of skeletal diseases. Robust osteogenic abilities not only enhance bone tissue regeneration and repair but also yield excellent effects in implant osseointegration. Typically, osteogenesis involves the migration of osteoblasts to the site where bone tissue synthesis occurs. They secrete and synthesize collagen and bone protein fibres, subsequently forming new bone tissue by absorbing calcium and phosphorus into the fibres’ pores for precipitation and crystallization [39,40]. Addressing the question of how to promote osteogenesis to enhance bone tissue regeneration represents the most valuable research in orthopaedics and stomatology. The exploration and application of biomaterials, particularly the development of multifunctionally smart implant materials, offer innovative strategies for advancing the healing process of bone tissue defect. In this section, we comprehensively summarize and illustrate the latest strategies and mechanisms for promoting osteogenesis through smart bone implants, focusing on their multifunctional and synergistic effects. These include enhancing osteogenesis, preventing infection, promoting angiogenesis, modulating the immune response, neurogenic regulation, and adapting osteogenic microenvironments (Fig. 2 and Table 1). By integrating these functions, smart bone implants enhance bone repair and regeneration, offering promising clinical applications in orthopaedic biomaterials.
Fig. 2.
Summative scheme of the latest multifunctional strategies and synergistic mechanisms of smart bone implants for enhanced bone repair and regeneration.
Table 1.
Osteogenic function and mechanisms of smart bone implants.
| Implant material | Osteogenic mechanism | Cell | Ref. |
|---|---|---|---|
| Regulation of osteogenesis-related signaling pathways | |||
| silica-coated GO/GelMA scaffold | adsorption and release of endogenous BMPs, activation of the BMPs/Smad signaling pathway, released silicon ions triggering the upregulation of BMP2 | BMSCs | [87] |
| nHA/GO/CS scaffold | mild photothermal effect, enhance the BMP2/Smad signaling pathway | MC3T3-E1 cells, human BMSCs | [89] |
| hydrogel delivery system | localized delivery and synergistic release of Mg2+ and Zn2+, upregulate MAPK signal pathway | human BMSCs | [93] |
| nano-Mg(OH)2 films on Ti surfaces | release Mg2+ and create a weakly alkaline microenvironment, promote TGF-β signal pathway | murine C3H10T1/2 cells | [94] |
| functionalized methacrylate hyaluronic acid hydrogels | activate the noncanonical Wnt signaling pathway, enhance intracellular calcium levels | human skeletal stem cells | [97] |
| Mg-1Ca/PCL scaffolds | releasing Mg2+, activate Wnt/β-catenin signaling pathway | human BMSCs | [96] |
| 3D-printed Mg-1Ca/PCL scaffolds | macro- and microstructures, releasing Mg2+, activating Wnt/β-catenin signaling pathway | human BMSCs | [96] |
| PCL polymer film bandage | deliver the WIOTM, asymmetric cell division, enhance β-catenin and Wnt signaling pathway | human SSCs | [98] |
| Balance bone homeostasis | |||
| Mn-TCP | release Mn2+ ions, scavenge ROS, promote osteogenic differentiation | RAW264.7 cells, mouse BMDMs | [101] |
| nanoporous Ti implant | downregulate the integrin-mediated β1/FAKpY397/MAPK pathway, micropitted/nanotubular topography | RAW264.7 cells, MC3T3-E1 cells | [102] |
| hyaluronic acid nanocomposite hydrogel | acidic environment-responsive release of the bisphosphonates, inhibit osteoclast maturation, enhance in situ bone regeneration | RAW264.7 cells, mouse BMMs, rat BMSCs | [103] |
| AdOPG mediated nanoparticulate mineralized collagen glycosaminoglycan scaffold | enhance BMP signaling pathway, uncoupling osteogenic and osteoclastogenic differentiation | human BMSCs, human osteoclasts | [106] |
| high Ca/P ratio CPC scaffold | promote secretion of endogenous TGF-β1 to recruit BMSC, enhance osteoclastogenesis | mouse BMMs, rat BMSCs | [108] |
| Regulation of autophagic flux | |||
| negative pressure hydrogels with BMSCs | promote AMPK-ULK1 (unc-51-like kinase 1)-autophagy axis | rat BMSCs | [115] |
| LDO nanosheet coated Ti | Release of Mg2+ and Ga3+, generate an appropriate alkaline microenvironment (about pH 8.5), promote AMPK and mTOR signaling pathways | rat BMSCs | [120]. |
| Sr-doped micro/nano rough Ti | upregulate Akt-mTOR-dependent signaling pathways, altered RANK/RANKL/OPG axis | rat BMSCs, rat BMDMs | [122]. |
| Biomaterials surface morphology enhance osteogenesis | |||
| nanotopographical titanium plates | release of pro-osteogenesis sEV (Ti4-21-sEVs) | human BMSCs | [129] |
| sEV decorated 3D-printed PEEK scaffold | time-dependent osteogenic activity, deliver osteogenic miRNAs | human BMSCs | [129] |
| exosomes modified 3D-printed Ti alloy scaffold | upregulate osteogenic miRNAs level, activate PI3K/Akt and MAPK signaling pathways | human BMSCs | [130] |
| micron-size micropillar arrays on Ti surface | modulate nuclear deformation, alter chromatin organization | human BMSCs | [131] |
| methacrylated poly(octamethylene citrate) micropillar implant | nuclear deformation, lamin A/C multimerization, alter chromatin conformation and reprogramming | human BMSCs | [132] |
| flower-like nanostructures CaSiO3 bioceramics | promote cell adhesion and ALP activity, enhance FAK/p38 signaling pathway | rat BMSCs | [135] |
2.1. Diverse patient-oriented osteogenic microenvironments
The term “osteogenic microenvironment” refers to the local biological milieu surrounding bone tissue, where a delicate interplay of bone metabolism-related cellular and signal molecules orchestrates the intricate processes of osteogenesis-the formation of new bone [41,42]. In clinical practice, patients with conditions such as osteoporosis, menopause, obesity, diabetes mellitus (DM), rheumatoid arthritis (RA), chronic kidney disease (CKD), and thyroid dysfunction often experience impaired bone tissue healing due to a disrupted osteogenic microenvironment [[43], [44], [45], [46], [47]]. Therefore, designing smart implants to meet the specific needs of patients undergoing bone repair becomes pivotal in the realm of orthopaedic treatments, as it profoundly ameliorates the osteogenic microenvironment compromised by various diseases to accelerate bone tissue regeneration.
One crucial consideration in achieving optimal osteogenic microenvironment is addressing bone density-related issues such as osteoporosis. For patients afflicted by senile osteoporosis, postmenopausal osteoporosis, and glucocorticoid-induced osteoporosis, they are mainly characterized by decreased bone mineral density, compromised skeletal strength, and trabecular microstructure, as well as an increased risk of fractures [[48], [49], [50]]. In the osteoporotic marrow, accumulating reactive oxygen species (ROS) and proinflammatory cytokines can create a chronic inflammatory state, which not only activates osteoclast precursors for promoting bone resorption but also suppresses the number of osteoblasts [51,52]. In cases where patients present with comorbidities such as estrogen, glucocorticoid, and parathyroid hormone (PTH) deficiency, the regulatory pathways governing bone metabolism are further disrupted in bone repair procession [53]. Estrogen deficiency, for instance, is associated with decreased osteoblast activity, while glucocorticoid excess can lead to impaired osteogenic differentiation and increased bone resorption (Fig. 3a) [54,55]. PTH deficiency may contribute to disturbances in calcium homeostasis, impacting bone mineralization [56]. Accordingly, in osteoporotic conditions, bone homeostasis is disrupted by excessive bone resorption and insufficient bone formation, achieving bone-implant osseointegration more challenging than under normal conditions. To devise targeted interventions in osteoporotic patients, a programmed PEEK surface with a degradable hybrid coating is designed to sequentially regulate osteoimmunomodulation and bone regeneration [57]. By initially releasing interleukin-4 (IL-4) to mitigate acute inflammation, followed by sustained delivery of alendronate and Ca2+ to enhance osteogenesis, suppress osteoclastogenesis, and ultimately improve bone-implant osseointegration under osteoporotic conditions. Similarly, Chen et al. constructed that the biofunctional metal-organic framework (bio-MOF) coating on the Ti implant surface, which led to improved osteointegration in the senescence-induced osteoporotic microenvironment [58]. The novel strategy effectively decomposed excessive ROS, restored mitochondrial dynamics, and reprogrammed senescent bone marrow mesenchymal stem cells (BMSCs) into normal BMSCs, ultimately recovering their osteogenic potential.
Fig. 3.
Mechanisms underlying bone remodelling imbalance in pathological microenvironment. (a) Overview of age-related changes in the bone marrow microenvironment. In young/healthy marrow, osteoblasts and osteoclasts are balanced via coupling agents such as RANKL, IGF-1, and PDGF-BB. In old/osteoporotic marrow, accumulating ROS and proinflammatory cytokines can create a chronic inflammatory state, finally leading to a bone matrix that becomes thin and porous (osteoporotic) and susceptible to fracture [55]. Copyright 2016, Elsevier Ltd. (b) Cellular and molecular mechanisms of bone diseases in diabetes mellitus [63]. Copyright 2017, Springer Nature.
Furthermore, diabetes mellitus is affected by a partial or complete failure of β-cells of the pancreas combined with low levels of insulin-like growth factor 1 (IGF1), which significantly suppresses the terminal differentiation of BMSCs into osteoblasts, along with a notable reduction in osteoblastic activity [59,60]. As diabetes progresses, the concurrent presence of hyperglycemia, chronic inflammation, advanced glycation end products, and microangiopathy, collectively exert detrimental effects on both the architecture and biomechanical properties of the skeleton (Fig. 3b) [[61], [62], [63]]. The microvascular complications significantly impair angiogenesis and microcirculatory dysfunction, and impede the necessary nutrient and oxygen delivery to the healing site, thereby affecting osteogenesis and bone-implant osseointegration. Given that vascularization is orchestrated by BMSCs-mediated osteogenic microenvironment, deficiencies in vasculature may be exacerbated during the persistent chronic inflammatory milieu characteristic of diabetes [64,65]. In addition to these impediments, patients living with diabetes mellitus face an elevated risk of periprosthetic joint infection (PJI). This heightened susceptibility is attributed to the complex interplay of factors such as compromised immune function, altered tissue perfusion, and the presence of chronic hyperglycemia, collectively creating an environment conducive to bacterial attachment and biofilm formation [66,67]. Tailoring therapeutic strategies to address both the inflammatory aspects, poor neovascularization, and the specific PJI of diabetes is crucial for promoting effective bone regeneration and mitigating the progression of osteoporosis.
Tightly regulated interactions between the immune and skeletal systems have reaffirmed that aberrant immune responses have a strong potential to govern bone metabolism within the osteogenic microenvironment [68]. As a chronic autoimmune disease, RA is characterized by persistent inflammation, predominantly affecting synovial joints [69]. The immune system's aberrant activation in RA results in the production of pro-inflammatory cytokines, such as tumour necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which have far-reaching negative effects on the local bone microenvironment via initiating the inflammatory cascade [70,71]. Furthermore, the dysregulated immune responses in RA can instigate the production of autoantibodies, such as rheumatoid factor and anti-citrullinated protein antibodies [72,73]. These autoantibodies not only contribute to the perpetuation of inflammation but also directly activate osteoclastogenesis and bone destruction [74,75]. Simultaneously, the chronic inflammatory state would alter the immune milieu, contributing to increased osteoclast activity by stimulating the production of receptor activator of nuclear factor-kappa B ligand (RANKL), a key regulator of osteoclastogenesis, by various immune cells [76]. In the context of RA, the differentiation and function of osteoblasts (OB) were inhibited by B cells producing chemokine (C–C motif) ligand 3 and TNF within the bone marrow microenvironment, contributing to bone loss and joint destruction [77]. The intricate interplay between the immune and skeletal systems is underscored by the impact of aberrant immune responses on the osteogenic microenvironment. RA serves as a paradigm, illustrating how chronic inflammation and immune dysregulation disrupt the delicate balance of bone metabolism, eventually leading to peri-implant bone loss and implant loosening [78]. Understanding these complexities is vital for developing targeted therapeutic interventions to mitigate implant-related complications in conditions characterized by immune dysregulation. Otherwise, with the advancement of society and technology, an increasing number of individuals with unhealthy habits such as drinking, smoking, and staying up late are showing varying degrees of inflammatory responses and weakened immunity. As a result, they exhibit reduced osteogenic ability compared to the general population, which draws more attention to the need for developing targeted implants with enhanced bone regeneration capacity.
The development of smart orthopaedic implants targeting the clinical patient-oriented osteogenic microenvironment represents a promising strategy for enhancing bone tissue regeneration. With the introduction of innovative implants integrating advanced materials and technologies, there is an effective achievement of self-adaptive prevention and treatment in response to bone repair under dynamic pathological conditions. Ultimately, we believe that the pursuit of an optimized osteogenic microenvironment in clinical practice will contribute to advancing patient outcomes, promoting faster and more durable bone healing, and improving the overall quality of life for individuals undergoing orthopaedic interventions.
2.2. Regulation of osteogenesis-related signaling pathways
Osteoblasts, primarily derived from BMSCs, stand as crucial cells in fostering the osteogenesis of the bone remodelling process in vivo [79]. The osteogenic differentiation of BMSCs undergoes regulation through multiple signaling pathways, including bone morphogenic proteins/sma and mad (BMPs/Smad) signaling pathway, mitogen-activated protein kinase (MAPK) signaling pathway, AMP-activated protein kinase (AMPK) signaling pathway, transforming growth factor (TGF) signaling pathway, wingless/integrated (Wnt) signaling pathway, and others [[80], [81], [82], [83], [84]]. These pathways collectively form a complex and precise regulatory network that plays a pivotal role in controlling osteoblast differentiation, osteogenesis, and bone metabolism. Numerous smart bioactive materials exhibit significant therapeutic potential by modulating osteogenic signaling pathways [85,86]. Consequently, smart implant materials are anticipated to demonstrate exceptional capabilities in promoting osteogenic differentiation, thereby contributing to the advanced healing process of bone tissue.
The BMPs/Smad signaling pathway works via the direct binding of BMPs to receptors on the surface of osteoblasts, initiating the bone formation process through the intracellular Smad signaling system [80]. An illustrative example involves the design of graphene oxide-functionalized methacrylate gelatin (GO/GelMA) scaffolds to enhance the osteogenic capability of BMSCs. These scaffolds enhance the adsorption and release of endogenous BMPs, synergistically cooperating with high activation of the Smad1/5 pathway for advanced osseointegration [87]. Similarly, the silica-coated GO/GelMA nanocomposite scaffolds further promote mineralization and osteogenesis through the released silicon ions triggering the upregulation of BMP2. In a mouse subcutaneous implantation model, the BMSC-loaded GO/GelMA and SiGO/GelMA samples exhibit remarkable increases in bone volumes, compared to the GelMA control group. In addition, the near-infrared (NIR) light irradiation has also been proven to enhance osteogenesis via the BMP2/Smad signaling pathway [88]. Ma et al. successfully designed a novel temperature-controlled nano-HA/graphene oxide/chitosan (nHA/GO/CS) scaffold, which exhibited excellent osteogenic ability of human BMSCs at 42 ± 0.5 °C under NIR irradiation [89]. With the introduction of nHA, the multifunctional scaffold improved the biocompatibility and biological activity, and further enhanced osteogenic differentiation via upregulated the BMP2/Smad signaling pathway. Furthermore, the endogenous proteins are used to modify gold nanorods, which improved biocompatibility and reduced immune response [90]. It is worth noting that modified gold nanorods with NIR laser irradiation may successfully stimulate osteogenic differentiation of BMSCs by enhancing the osteogenic signal transduction pathways, including MAPK, Akt, Smad, and β-catenin. Surprisingly, tobacco mosaic virus, turnip yellow mosaic virus, and other plant viruses modified gold nanorods for promoting osteogenic differentiation of BMSCs by the elevation of BMP-2 gene expression in an autocrine way, as reported by Metavarayuth et al. (Fig. 4a) [91]. All of these materials, notwithstanding their differences, significantly induce the expression of the BMP2 signaling pathway for advanced osteogenesis.
Fig. 4.
Multi-dimensional analysis of osteogenesis and bone regeneration mechanisms. (a) Proposed pathway in stem cells involves BMP-2 intermediates osteogenesis in which initiated by unique nanoscale topography of virus substrate [91]. Copyright 2019, American Chemical Society. (b) Potential molecular signaling pathways by which nano-Mg(OH)2 films on Ti surface influence cell behaviours [94]. Copyright 2021, Elsevier Ltd. (c) Schematic representation of the possible cell division modes in three dimensions on a Wnt3a surface. (d) Representative confocal Z planes with 3D reconstructions and side-view projections of hSSCs undergoing perpendicular cell division at the Wnt3a-platform. The cells were stained with antibodies against APC (cyan) and β-catenin (magenta) and the nuclei were stained with DAPI (yellow). (e) Representative confocal z planes of the base (cells contacting the Wnt3a-platform, middle (10–50 μm from the platform) and top (50–100 μm from the platform) of the WIOTM after immunostaining against CDH13 (cyan) and OPN (magenta) and with DAPI (yellow). (f) Representative micro-CT images of calvarial defects in mice at 8 weeks post-procedure [98]. Copyright 2021, Springer Nature.
Previous studies demonstrated that magnesium ions (Mg2+) promotes the osteogenic differentiation of BMSCs via activation of the MAPK signaling pathway, and increased osteoblast activity and mineralization capacity [83,92]. The smart hydrogel delivery system, for example, incorporated a rationally designed T4 lysozyme mutant (T4M) with cross-linked bioactive ions to mediate the localized delivery and synergistic release of Mg2+ and zinc ions (Zn2+), which promoted osteogenic differentiation of BMSCs and bone regeneration through significantly increasing the expression of MAPK pathway-related genes [93]. Moreover, Yao et al. developed two bioactive nano-Mg(OH)2 films on Ti surfaces using hydrothermal treatment [94]. As shown in Fig. 4b, the Mg(OH)2 films, as a nano-sheet-like structure, can release Mg2+ and create a weakly alkaline microenvironment surrounding the implant, which lead to improved osteogenesis via extracellular matrix (ECM) receptor interaction, focal adhesion, and promotion of BMP-4 expression by activating the classic TGF-β signal pathway.
The Wnt signaling pathway is intricately involved in the regulation of bone homeostasis, and bispecific Wnt mimetics such as 18R5-DKK1c have demonstrated the ability to stimulate bone repair and remodelling [81,95]. Recent studies have demonstrated that three-dimensional (3D)-printed Mg-1Ca/polycaprolactone (PCL) composite scaffolds with precisely controlled macro- and microstructures significantly promote new bone formation and effectively repair bone defects [96]. These scaffolds achieve their osteogenic effects by stably releasing Mg2+ ions, which facilitate BMSCs adhesion and proliferation, and by activating osteogenic differentiation through the Wnt/β-catenin signaling pathway. The functionalized methacrylate hyaluronic acid hydrogels containing the Foxy5 peptide, a noncanonical Wnt5a mimic, stimulate the osteogenic differentiation of human skeletal stem cells (hSSCs) by not only activating the noncanonical Wnt signaling pathway involving RhoA-ROCKs but also boosting intracellular calcium levels [97]. In addition, Okuchi et al. covalently bound Wnt3a onto microbeads and an aldehyde-modified glass platform (Wnt3a-platform) in a 3D environment of collagen type 1 gel, where BMSCs were co-cultured and subsequently formed an organized 3D Wnt-induced human osteogenic tissue model (WIOTM) [98]. Then, they designed a biodegradable PCL polymer film bandage to deliver the WIOTM (WIOTM-bandage) to integrate and contribute to bone repair. The localized application of Wnt3a directs the asymmetric cell division of BMSCs, resulting in the generation of two distinct populations: Wnt3a-distal cells, characterized by an enrichment of early osteogenic differentiation genes, and Wnt3a-proximal cells, exhibiting elevated levels of β-catenin, aPKCζ (a cell polarity protein), and various stem cell markers. This orchestrated division serves to maintain the quantity of human stem and osteogenic cells at the injury site. Moreover, it significantly augments the activation of the β-catenin pathway, thereby expediting endogenous osteogenesis and efficient bone repair (Fig. 4c–f). Therefore, the mechanism by which the Wnt signaling pathway regulates osteogenesis has been revealed and represents a viable strategy of biomaterials functionalized with related signaling proteins to contribute to bone repair.
The different signaling pathways play critical roles in the osteogenic differentiation of BMSCs and osteogenesis, thereby, smart implants embedding biomolecules (e.g., signaling pathway proteins, growth factors, etc.) or other biodegradable ingredients provide novel insights into the treatment of bone defect regeneration. However, novel biomaterials lead to many challenges such as biocompatibility, biological toxicity, immunogenicity of bioactive molecules, and other diseases induced by intracellular signaling activating mutations, etc. These problems limit the application and clinical translation of the innovatively smart implants, but the biofunctionalization of biomaterials may offer future effective therapeutic approaches for bone tissue regeneration and repair.
2.3. Dynamic balance between osteoblastogenesis and osteoclastogenesis
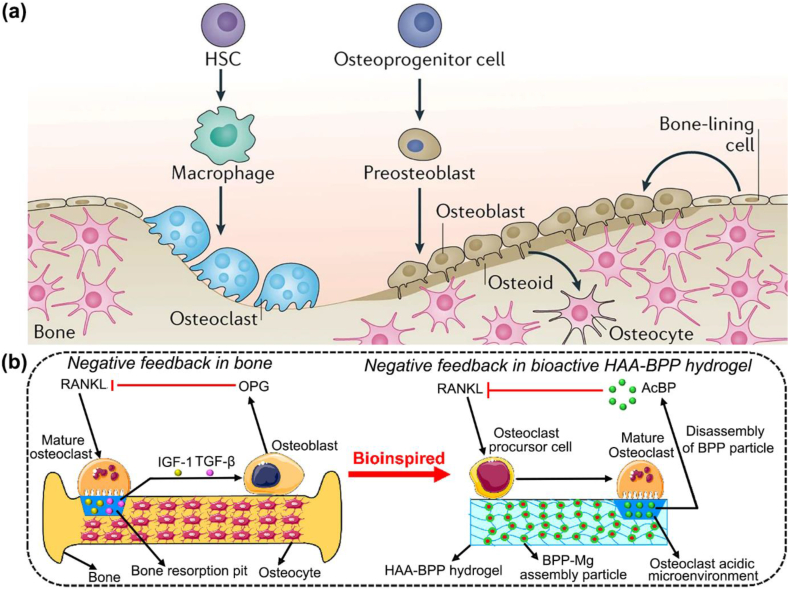
The dynamic maintenance of bone structure, i.e., bone homeostasis, involves a delicate equilibrium between bone formation by osteoblasts and resorption by osteoclasts. Osteoclasts, originating from the hematopoietic lineage of monocytes or macrophages, predominantly govern the resorption of bone matrix and mineralized tissue through alterations in the receptor activator of nuclear factor-κB (RANK), RANKL, and osteoprotegerin (OPG) axis. This regulatory mechanism plays a crucial role in osteogenesis and bone homeostasis (Fig. 5a) [99]. Simultaneously, the release of TGF-β and IGF1 follows the bone resorption process, triggering osteoblast differentiation and the formation of a new bone matrix [100]. Therefore, the reasonable regulation of resorptive osteoclast activity to maintain bone homeostasis stands as a significant strategy for promoting osteogenesis and bone defect repair.
Fig. 5.
Mechanisms of bone homeostasis and biomimetic regulation of osteoclast activity. (a) Schematic illustration of the bone homeostasis achieved through the balance between the activity of osteoblast lineage cells and osteoclast lineage cells. Osteoblast lineage cells such as the osteoid secrete HA and calcium to promote bone mineralization and formation of osteocytes. Meanwhile, osteoclast lineage cells resorb the bone tissue. HSC is the hematopoietic stem cell [99]. Copyright 2020, Springer Nature. (b) Schematic flow of the native negative feedback regulation of osteoclasts in bone and the biomimetic emulation of the negative feedback regulation of osteoclasts by a bisphosphonate-based nanocomposite hyaluronic acid (HAA-BPP) hydrogel. Osteoclastic activities degrade the HAA-BPP hydrogel, thereby triggering the release of BPP, which in turn inhibits RANKL-induced osteoclastic maturation [103]. Copyright 2022, Elsevier Ltd.
To inhibit osteoclast differentiation and osteoclastic activity for treating bone tissue regeneration and osteoporosis is an effective strategy. The Mn-contained β-tricalcium phosphate (Mn-TCP) bioceramics are successfully fabricated to accelerate bone tissue regeneration in osteoporotic rats via promoting osteogenic differentiation of osteoblasts and decreasing the formation and resorptive function of osteoclasts [101]. The related detailed mechanism is that the released Mn2+ ions scavenge ROS by activating nuclear factor-erythroid 2-related factor 2 to increase antioxidant enzymes, ultimately decreasing the number of osteoclasts. Similarly, He et al. revealed that nanoporous titanium implant surfaces, such as the micropitted/nanotubular topography and micropitted topography, significantly accelerating early bone formation and osseointegration around the implant in vivo [102]. The nanoporous surface could remarkably inhibit the formation of osteoclasts by significantly downregulating the integrin-mediated β1/FAKpY397/MAPK pathway, thus relieving the inhibition of osteoclasts on osteogenesis via altering the secretion of macrophage cytokine profiles and clastokines. In addition, the smart stimuli-responsive biomaterial scaffolds provide valuable inspiration for facilitating osteogenesis and bone regeneration. Li et al. designed the biomimetic hyaluronic acid nanocomposite hydrogel by coordination bonds with the antiosteoclastic drugs bisphosphonates [103]. Under the acidic environment created by mature osteoclasts secreting abundant amounts of hydrogen ions into the subosteoclast compartment [104], the biomimetic hydrogel exhibited the expedited release of loaded bisphosphonates and then the osteoclast maturation. However, this hydrogel only releases basal levels of bisphosphonates in the presence of preosteoclasts, whereas it releases a significant number of bisphosphonates when mature osteoclasts are present. This conclusion may help to selectively inhibit mature osteoclasts through biomimetic negative feedback regulation, which would improve osteogenesis and bone repair in vivo.
Under bone homeostatic conditions, osteoclastic activity is regulated by negative feedback. The activity of mature osteoclasts is increased with increasing RANKL exposure, resulting in the release of matrix-bound factors such as IGF1 and TGF-β, subsequently triggering the recruitment of osteoblasts and synthesis of OPG, which can inhibit RANKL-induced osteoclast maturation (Fig. 5b) [99,103,105]. The nanoparticulate mineralized collagen glycosaminoglycan scaffolds can induce osteogenic differentiation via through activation of the canonical BMP signaling pathway. Then, the special scaffolds are integrated with adenovirus-mediated delivery of OPG (AdOPG), an endogenous anti-osteoclastogenic decoy receptor, uncoupling osteogenic and osteoclastogenic differentiation that inhabit osteoclast resorptive activity to enhance osteogenesis [106]. This reveals that the regulation of osteoclast resorptive activity while maintaining the positive paracrine effects on osteogenic differentiation is a new avenue for the development of osteogenic biomaterials. When cytokines are activated at the implant interface, osteoclasts play a crucial part in osseointegration, whereas any suppression of osteoclastogenesis may delay bone repair [107]. Nowadays, CaP-based materials, such as calcium phosphate cement (CPC), dicalcium phosphate (DCP) bioceramic scaffolds, etc., have been demonstrated that could modulate osteoclast-mediated osseointegration efficiently [108]. The CPC scaffolds with high Ca/P ratio can significantly enhance osteoclastogenesis and activity via increasing affinity between RANKL and RANK, meanwhile promoting the secretion of endogenous TGF-β1 to recruit BMSCs and significantly ameliorate osseointegration. This perspective diverges from the conventional notion that inhibiting osteoclastogenesis promotes bone formation. Instead, it establishes a connection between osteoclasts and osteoblasts, introducing a novel strategy to promote osteogenesis and advance bone repair.
Furthermore, the few-layered Nb2C (MXene) is also recently demonstrated to effectively inhibit osteoclastogenesis by its ROS adsorption ability [109,110]. Other researchers found that selenoprotein W acts as an osteoclastogenic stimulator and engages in negative feedback to suppress osteoclast differentiation through downregulating RANKL/RANK/TNF receptor-associated factor 6/p38 signaling [111]. In addition, the antiosteoclastic nano-biomaterials or specific signaling molecules may be more efficient in reducing osteoclastogenesis and triggering osteogenesis via local nanoparticle drug delivery systems. The preceding multifarious biomaterials give innovative methods for promoting osteogenesis by reducing osteoclast differentiation and maturation. Nevertheless, to achieve long-term osteogenesis, future emphasis should be placed on the moderate regulation of osteoclast activity and its close interaction with osteoblasts.
2.4. Regulation of autophagic flux in osteogenesis
Autophagy, a major catabolic process in eukaryotic cells that degrades and recycles damaged macromolecules and organelles, contributes to mediating the survival and functioning of osteoblasts, osteocytes, and osteoclasts, which plays an essential role in bone homeostasis [112,113]. In addition, the levels of autophagy are enhanced during osteoblast differentiation of BMSCs through AMPK signaling pathways [113], and autophagic vacuoles could be used as vehicles to secrete apatite crystals during the mineralization process [114]. This also reveals the link between autophagy and osteogenesis, which provided a new strategy to improve the treatment of nonunion fractures. According to the report of Zhang et al., the combination therapy using BMSCs in hydrogels with negative pressure greatly boosted osteoblast differentiation and bone regeneration by identifying a novel AMPK- unc-51-like kinase 1 (ULK1)-autophagy axis [115].
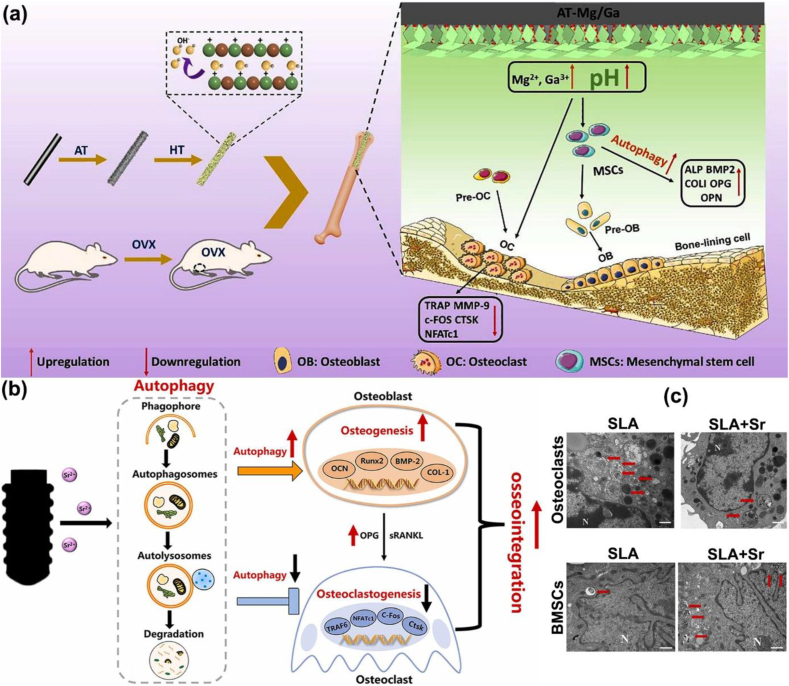
As a novel bioactive inorganic material, calcium silicate (Ca2SiO4) nanoparticles (NPs) have been identified as a potential candidate for bone repair [116], which could up-regulate autophagy by activating mammalian target of rapamycin (mTOR)/ULK1 and subsequently triggering the Wnt/β-catenin pathway to boost the differentiation and mineralization of osteoblasts [117]. Previous studies have illustrated that the alkaline microenvironment around the repair and regeneration of bone tissue induces autophagy as a cytoprotective mechanism and decreases the activity of osteoclasts to promote osteogenesis [118,119]. Chen etc. successfully fabricated the coating with Mg-Ga layered double oxide nanosheets, which was in situ grown on the surfaces of alkali-heat-treated titanium (AT-Mg/Ga) implants (Fig. 6a) [120]. To our surprise is that the novel coating displayed good stability and generated an appropriate alkaline microenvironment (about pH 8.5) over the long term due to the Mg2+ and Ga3+. Under the resulting weak alkaline environment, significantly improved the autophagic level by regulating AMPK pathways via P2Y receptors and activating ULK1 to trigger mTOR signaling pathways, thus promoting the osteogenic differentiation of BMSCs and suppressing osteoclastogenesis to achieve interfacial osseointegration.
Fig. 6.
Mechanisms of autophagic regulation in bone remodelling. (a) Schematic illustration of the AT-Mg/Ga implants that promoted autophagic activity that upregulated the osteogenic differentiation level of BMSCs and suppressed osteoclastogenesis to achieve interfacial osseointegration. (HT: hydrothermal treatment; OVX: ovariectomy) [120]. Copyright 2022, Elsevier. (b) Schematic diagram of the regulatory role of autophagy in osteogenesis and osteoclastogenesis stimulated by Sr-doped titanium implant. (c) TEM images of the autophagosomes in osteoclast and BMSCs seeded on the SLA and SLA + Sr surface for 24 h (N: cell nucleus; red arrow: autophagosome) [122]. Copyright 2022, Elsevier.
Furthermore, the titanium nanotopography has been reported to regulate cytoplasmic Yes-associated protein (YAP, an effector protein involved in the degradation of β-catenin) degradation and activate the β-catenin pathway to induce osteoblast autophagy, which can promote osteogenic differentiation and mineralization [121]. Wang etc. fabricated Sr-doped micro/nano rough titanium implant surface by sandblasted, large-grit, acid-etched (SLA) and following hydrothermal treatments (SLA + Sr) that endowed implants with the ability to modulate autophagy to promote osteogenesis and osseointegration (Fig. 6b) [122]. The distinctive mechanism underlying the Sr-incorporated micro/nano-rough titanium implant surface involves the synergistic effects of nano-morphology and Sr2+, leading to the upregulation of osteogenesis and autophagy through Akt-mTOR-dependent signaling pathways. Simultaneously, this modification enhances the secretion of OPG by osteoblasts, thereby suppressed osteoclast autophagy and osteoclastogenesis via influencing the RANK/RANKL/OPG axis (Fig. 6c). Concurrently, the innovative modified Ti significantly accelerated bone tissue regeneration and improved periprosthetic osseointegration. This interaction between autophagy and osteogensis signifies a promising avenue for smart implant design, where the intricate cellular reprogramming processes hold the key to enhance osseointegration and long-term implant success.
Nowadays, researchers also confirm that the upconversion optogenetic nanosystem could up-regulate autophagy via converting tissue-penetrative NIR light into local visible blue light [123], which provides the possibility of modulating autophagy in combination with photodynamic therapy to promote osteogenesis. Hence, the regulation of autophagy is a crucial pathway for promoting osteogenesis, especially the role of an alkaline environment, which reveals a new horizon for designing appropriate implant materials to enhance their osseointegration in the future translational medicine field.
2.5. Osteogenic activity of the surface topographies of implants
For smart bone implants, the optimization of topography and morphological characteristics of synthetic biomaterial surfaces is robustly demonstrated as an effective approach to enhance their biological performance, offering novel insight and potential mechanism for bone tissue regeneration and repair. Surface modifications have been employed to establish favourable biological interfaces, augmenting the interfacial bioactivity of bone-implanted biomaterials [124]. Herein, we systematically summarize and discuss the diverse osteogenic surface properties of biomaterials along with their underlying mechanisms.
Micro/nano-textured topography structures show favourable roughness, hydrophilicity, and mechanical interlocking ability, which endow the biomaterials capable of guiding cell adhesion, proliferation, and differentiation [125]. Zhang et al. demonstrated that micro/nanonet-textured hierarchical titanium topography enhanced the spreading areas of BMSCs on its surface and boosted exosomes synthesis and transport by upregulating RAB27B and sphingomyelin phosphodiesterase 3 gene expression [126]. Exosomes released by BMSCs have been demonstrated in vivo and in vitro to promote BMSC adhesion, proliferation, and osteogenic differentiation, as well as to allow enhanced bone regeneration by activating the phosphatidylinositol 3-kinase (PI3K)/Akt pathway or suppressing the plexin B1/RhoA (ras homolog gene family, member A)/ROCK (Rho-associated kinase) pathway [127,128]. Therefore, this reveals a potential mechanism of the implant surface topographies that can regulate the function of exosomes and thereby promote BMSCs osteogenesis differentiation, which also expands new horizon for the development of bioactive smart materials. The nanotopographical titanium plates are produced through alkali heat treatment, which can stimulate human BMSCs (hBMSCs) osteogenic differentiation and cause the release of pro-osteogenesis small extracellular vesicles (sEVs, Ti4-21-sEVs) (Fig. 7a) [129]. Researchers discover that the sEVs secreted from hBMSCs cultured on Ti4 could be utilized to decorate 3D-printed polyether ether ketone (PEEK) scaffolds with polydopamine (PDA), and these scaffolds demonstrated enhanced osteogenic activity in a time-dependent manner without adding cells or other biochemical factors. Simultaneously, the engineered Ti4-21-sEVs significantly promoted osteogenesis in vivo and in vitro by delivering miRNAs (e.g., miR-497-5p and miR-210-3p) with osteogenic signaling pathways (Fig. 7b and c). Similarly, Zhai et al. discovered that exosomes extracted from hBMSCs at different stages of osteogenic pre-differentiation were used to modify 3D-printed Ti alloy scaffolds, enhancing osteogenesis and bone tissue regeneration by activating the PI3K/Akt and MAPK signaling pathways [130]. Therefore, the bioscaffolds bind to osteogenic exosomes, especially the exosomes induced by nanotopological surfaces, which provides a novel cell-free osteogenesis method for bone repair.
Fig. 7.
Osteogenic activity driven by the surface topographies of the implant. (a) Schematic of nanotopography regulation of mesenchymal stem cell sEVs that promote osteogenesis and 3D-printed PEEK scaffolds decorated with sEVs for bone regeneration; Osteogenesis indicators including OPN, RUNX2, ALP, and OCN were detected by RT-qPCR after incubation with 50 μg/mL Ti-21-sEV and Ti4-21-sEV for (b) 14 days and (c) 21 days, and untreated cells denoted as a control group. (∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001) [129]. Copyright 2021, American Chemical Society. (d) Representative false colored scanning electron micrographs highlighting phenotypic differences between BMSCs cultured on (i) flat substrates (control) and (ii) micropatterned substrates. (e) Representative false-colored FIB/SEM image of BMSCs cultured on 5 × 5 substrate. Micropillars are colored red, with nuclei colored blue [131]. Copyright 2021, Wiley-VCH GmbH. (f) Schematic illustration of the influence of contact-guidance-induced nuclear deformation on bone regeneration. Microtopography engineering was used to create the micropillar implants fabricated using methacrylated poly(octamethylene citrate) (mPOC) [132]. Copyright 2023, Springer Nature.
The micron-size micropillar arrays are also prepared by ultraviolet nanoimprint lithography and exhibit excellent osteogenic capacity of BMSCs, especially on micropillars with 5 μm width/spacing and height [131]. This reveals new insight into promoting osteogenesis by microscale structures, which modulate nuclear deformation caused by myosin-II-generated tension on the actin cytoskeleton and subsequently alter chromatin organization and gene expression (Fig. 7d and e). More importantly, Wang et al. innovatively revealed that the micropillar-induced nuclear deformation could facilitate bone regeneration via chromatin engineering method, as shown in Fig. 7f [132]. The methacrylated poly(octamethylene citrate) micropillars profoundly influenced nuclear architecture, lamin A/C multimerization, and chromatin reprogramming through contact-guidance-induced nuclear constriction. These chromatin conformation changes collectively facilitate osteogenic differentiation of hBMSCs and bone tissue regeneration in mice with critical-size cranial defects. Similarly, titanium dioxide (TiO2) nanotube arrays with manganese-containing bioglass also exhibit higher bone formation and osseointegration capacity [133]. Moreover, Bai et al. fabricated TiO2 nanotube arrays with different diameters on titanium surfaces that exhibited excellent osteogenesis ability [134]. The nanotube arrays tends to induce the formation of clot made of thin fibres via coagulation protein adsorption, and subsequently regulate a favourable osteoimmunomodulatory environment with the release of growth factors, which can significantly accelerate the formation of bone healing hematoma, leading to better bone regeneration and osseointegration.
Moreover, the flower-like nanostructures were successfully constructed in situ on biodegradable calcium silicate (CaSiO3) bioceramics via hydrothermal treatment, which exhibited strong abilities to promote bone regeneration in vivo [135]. Compared with CaSiO3 bioceramics, the novel nanostructures significantly provide more sites for BMSCs adhesion and proliferation, which further activate the FAK/p38 signaling pathway to enhance osteogenic differentiation and bone regeneration. In addition, biological synthesis of the micro/nanostructures on bioceramics such as the mineralization in bacteria, is also a novel strategy to fabricate the advanced biomedical materials for bone tissue regeneration [136]. The microbially catalyzed micro/nanostructures can stimulate adhesion, spreading, and osteogenic differentiation of BMSCs by upregulating integrin alpha-2 and the Wnt signaling pathway to significantly enhance in vitro and in vivo osteogenesis.
Therefore, the achievement of multifarious micro/nanotopographical structures of bone implants through surface modification represent a novel and effective approach to promoting osteogenesis. This strategy can not only efficiently enhance the adhesion of BMSCs on the interface between bone and implants, but also actively stimulate the secretion of osteoinductive factors that subsequently activate corresponding osteogenic signaling pathways and then contribute to improved osseointegration. The diverse topographical surfaces offer valuable inspiration and novel insights into the design and production of bioactive materials for bone tissue regeneration and repair. In clinical practice, micro/nanotopographical surface modifications have been successfully applied to orthopaedic implants to enhance osseointegration and long-term stability, showing excellent prospects for clinical translation [137]. Notably, biodegradable magnesium-based implants with bioactive coatings have demonstrated promising clinical outcomes. For example, Mg-Nd-Zn-Zr alloy screws with calcium phosphate (Ca-P) coatings have demonstrated excellent osteoinductivity and biocompatibility in preliminary clinical trials for treating medial malleolar fractures, as evidenced by satisfactory fracture healing, gradual in vivo degradation, and improved postoperative functional recovery without severe complications [138]. However, in the context of these excellent biomaterial clinical applications, attention should also be given to the mechanical properties of micro/nanotopographical structures and the potential generation of wear particles, which may induce complications such as bone resorption and related osteolytic effects.
3. Bifunctional platform-based smart bone implants
Although exceptional osteogenesis and regenerative effects can be obtained with different biomaterials, several challenges remain to be addressed. For example, while the rough surface structure or nanotopography of bone implants plays a critical role in enhancing BMSCs adhesion and osteogenic differentiation, it may also unintentionally promote microbial attachment and biofilm formation on implant surfaces [139,140]. Additionally, a favourable osteoimmunomodulatory microenvironment can accelerate the transition from the initial inflammatory response to bone remodelling phase, highlighting the crucial role of immune modulation in promoting osteogenesis [19]. Finally, neovascularization plays a pivotal role in osteogenesis by ensuring adequate oxygen and nutrient supply to the regenerating bone tissue, thereby creating a microenvironment conducive to the recruitment of osteoprogenitor cells and facilitating bone formation and remodelling [141]. However, the synergistic interplay between osteogenesis and other critical functions, such as antibacterial, immunomodulatory, or angiogenesis, remains insufficiently explored in the design of osteogenic biomaterials. Therefore, bifunctional platform-based smart implants should be designed to integrate both osteogenesis with one of these additional functions, addressing multiple aspects of bone repair and regeneration simultaneously. In the following section, we summarize and illustrate various strategies for achieving this synergistic effect of bifunctionality in smart bone implants (Table 2).
Table 2.
Bifunctional platform-based smart implants.
| Biomaterial type | Smart composition | Advantage |
Model | Ref. | |
|---|---|---|---|---|---|
| Common | Special | ||||
| Antibacterial capacity and osteogenesis | |||||
| 3D hybrid scaffold | TDN, clindamycin | 1) destroy bacterial membranes and biofilms, 2) increase adhesion, spreading, proliferation, and osteogenic differentiation of BMSCs, 3) enhance the expression of osteogenesis-related genes and proteins, 4) reinforce osteoinductivity and osseointegration |
sustained release, enhance drug sensitivity | MRSA, rat BMSCs | [147] |
| 3D porous PEEK scaffold | Li+, antimicrobial peptide, porous structure | penetrate the bacterial cell wall | S. aureus, rat BMSCs | [149] | |
| surface modified Ti | ZnO/Zn3(PO4)2 hybrid nanostructures | physically rupture the bacterial membranes, release Zn2+, and generate ROS | S. aureus, E. coli, human BMSCs | [159] | |
| surface modified Ti | Al2O3-wrapped nanorod patterned array | mechano-puncture, | S. aureus, rat BMSCs | [151] | |
| surface modified Ti | PDA, Zn2+, nanorod arrays | physical puncture bacteria, enhance Fenton-like reactions, scavenge ROS, | S. aureus, E. coli, MC3T3-E1 cells | [157] | |
| Zn-Ag alloy implants | Zn2+, Ag+ | biodegradable, autolysis-related pathways, anti-osteolysis | S. aureus, Staphylococcus epidermidis, MRSA, MRSA 287, MC3T3-K cells, BMDMs | [161] | |
| surface modified Ti6Al4V | HA/MoS2, electrons transfer | disrupt redox balance, upregulate Ca2+ level, alter mitochondrial membrane | S. aureus, E. coli, MRSA, P. aeruginosa, rat BMSCs | [164] | |
| surface modified anodized Ti | triboelectric nanogenerator, negatively charged surface, electrical stimuli | reduce bacterial adhesion | S. aureus, E. coli, MC3T3-E1 cells | [167] | |
| 3D-printed scaffold | BPs, Zn2+, HA, photothermal effects | gradually release, Increase bacterial intracellular ATP and ROS levels, protein leakage, | S. aureus, E. coli, human BMSCs | [176] | |
| surface modified magnesium alloy | FeOOH nanosheets, Mg2+, photocatalytic and photothermal effects | corrosion resistance, sustained release | S. aureus, MC3T3-E1 cells | [177] | |
| surface modified Ti screw | photosonotherapy, ROS, | electron−hole separation, oxygen deficiency, | S. aureus, Porphyromanus gingivalis, MC3T3-E1 cells | [180] | |
| surfaced modified Ti | S-nitrosoglutathione, NO, photothermal therapy | controlling release of NO | S. aureus, E. coli, rat osteoblasts | [185] | |
| surface modified Ti | Mg/Zn-MOF74, Zn2+, Mg2+, | create alkaline microenvironment, anti-inflammatory | S. aureus, E. coli, rat osteoblasts, RAW264.7 cells | [7] | |
| 3D printed bioactive glass scaffold | FeSAC, •OH, hyperthermia | Fenton catalytic activity, destruct osteosarcoma | S. aureus, E. coli, human BMSCs, saos-2 cells | [191] | |
| surface modified Ti | LUT, Ca2+, PO43−, thermotherapy | acidic environment releasing | S. aureus, rat osteoblasts | [193] | |
| Immunomodulatory activity and osteogenesis | |||||
| injectable silk scaffolds | sitagliptin | 1) induce M2 phenotype macrophages polarization 2) ameliorate inflammatory microenvironment 3) promote osteointegration |
recruit M2 macrophages | rat osteoblasts, rat BMDMs | [217] |
| 3D-bioprinted scaffold | BMP-4 | sustained release, secrete BMP-2 | rat BMSCs, RAW264.7 cells | [220] | |
| surface modified Ti | honeycomb-like TiO2 structures | upregulate RhoA/ROCK signaling pathway | rat BMSCs, RAW264.7 cells | [33] | |
| surface modified Ti | Sr-doped Na2TiO3 nanorods arrays, Na+, Sr2+ | long-term release, paracrine of TGF-β1 and BMP2, enhance adhesion and filopodia formation of macrophage | MC3T3-E1 cells, RAW264.7 cells | [223] | |
| CS bioceramics | CaSiO3 | upregulate macrophage-derived OSM, upregulate ERK1/2 and JAK3 pathways | mouse BMDMs, mouse BMSCs | [226] | |
| tricalcium silicate cements | tricalcium silicate | secret BMP2, TGF- β1, and VEGF | mouse BMDMs, mouse BMSCs | [227] | |
| nanocomposite membrane | BaTiO3 | endogenous negative electrical microenvironment, inhibit PI3K/Akt signaling pathway | human monocytic THP-1 cells, human BMSCs | [232] | |
| nanocomposite membrane | CoFe2O4, P(VDF-TrFE), RGD-integrin | magnetoelectric microenvironment, activate PI3K/Akt signaling | rat BMSCs, RAW264.7 cells | [239, 242] | |
| delivery composite hydrogel | blood clot, BMP-2, hyperthermia effects | recruit macrophages, upregulate osteogenic protein expression | MC3T3-E1 cells, Balb/c mice cranial defect model | [245] | |
| surface modified Ti | PDA@HA nanorod-like array, photo-thermal therapy | ROS scavenging, sequential immunomodulation, release HSP 70, activate PI3K/Akt signaling pathway | mouse BMDMs, mouse BMSCs, RAW264.7 cells | [250] | |
| Angiogenesis and osteogenesis | |||||
| bioceramics | micro-nano-hybrid HAp and Sr2+ | 1) enhanced expression of osteogenic and angiogenic genes 2) improved expression of angiogenic factors 3) enhance vascularized bone regeneration |
micro-nanostructured surface promotes gene expression of osteogenic and angiogenic factors | rat BMSCs, | [275] |
| surface modified Ti6Al4V | magnesium-coated porous Ti6Al4V scaffold | improve cell proliferation, adhesion, ECM mineralization and ALP activity | MC3T3-E1 cells, HUVECs cells | [278] | |
| ion-Based Microspheres | Mg2+ and Si4+ | precise and controlled release of ions, massive collagen secretion | rat BMSCs, Ex vivo cam model (fertilized chicken embryos) | [281] | |
| 3D printed bioactive glass scaffolds | Nb2C MXene, calcium and phosphate released | biodegrades, photothermal ablation of osteosarcoma | Saos-2 cells (human osteosarcoma tumor cells), hBMSCs, HUVECs | [284] | |
| MgSnZnNa alloy | Na+, Mg2+ | co-release of Na and Mg ions | MC3T3-E1 cells, mouse adipose derived mesenchymal stem cells | [286] | |
| nanocomposite scaffolds | umbilical MSCs exosomes, HA-Gel, nanohydroxyapatite | exosomal miR-21 suppress the NOTCH1/DLL4 signal pathway, | umbilical MSCs, HEK293 cells, EPCs, and rat BMSCs | [300] | |
| β-TCP composite scaffolds | modularized sEVs | target ECs, BMSCs, and pre-OCs, inhibit OC maturation | mouse BMDMs, mouse BMSCs, HUVECs | [304] | |
| 3D printed PCL composite scaffolds | wesselsite [SrCuSi4O10] nanosheets, photothermal effect, Sr, Cu, and Si ions | thermal ablation of deep-seated osteosarcoma, | rat BMSCs, HUVECs, Saos-2 cells | [310] | |
| nanoparticle-hydrogel composite | mild magnetocaloric effect, cobalt ions | facilitated the expression of heat shock protein (HSP) 90, activates the PI3K/Akt pathway, | rat BMSCs, HUVECs | [311] | |
| nanofibrous scaffold | DMOG, BFP, simulate bone microenvironment | Activate PI3K/PKB/HIF-1α pathway, dual-drug delivery | rat BMSCs, HUVECs | [312] | |
| 3D printed enzyme-functionalized scaffold | Gox, CaP@CAT NSs, Ca2+ and PO43− ions | induce hypoxic microenvironment, alleviate the hyperglycemia environment | rat BMSCs, HUVECs, RAW264.7 cells | [322] | |
3.1. Synergistic interaction between osteogenesis and antibacterial/bactericidal capacity
Biomedical implant-associated infection is one of the most frequent and problematic complications related to the use of biomaterials, and the common routes of infection are intraoperative and hematogenous spread [67,142]. The rough structure of the biomaterial formed by surface modification not only improves BMSCs adhesion but also attracts bacteria at the implant-tissue interface, which can finally produce a biofilm that shields the bacteria and facilitates infection persistence [139]. As the concept of “race for the surface” is introduced, bacteria and host tissue cells compete for colonization on the surface of implanted materials, and in turn affects the osseointegration of the implant with the surrounding tissue, resulting in premature implantation failure [143]. Whatever the mechanism is, the design of multifunctional biomaterials to accomplish the antimicrobial effects and simultaneously promote cell affinity and osteointegration is a crucial solution to preventing implant infection.
3.1.1. Antimicrobial agent loaded bioactive implants
As the most traditional antibacterial method, an antibacterial agent combined with novel biomaterial could achieve the purpose of bacteria-killing by releasing the loaded agent into the surrounding tissues and its pharmacological effects [144]. Nowadays, the tetrahedral DNA nanostructure (TDN) are versatile 3D framework consisting of single-stranded DNA molecules, which may deliver medications while regulating their release and prolonging their effective concentration [145,146]. A 3D GelMA hybrid scaffold loaded with TDN and clindamycin complexes is fabricated, which representes exceptional biocompatibility, osteogenic and antibacterial activity, and significantly accelerates the repair of infected bone defects [147]. With the antimicrobial agents diffusing out of the hydrogels, the TDN-clindamycin-loaded scaffold significantly enhances the drug sensitivity of methicillin-resistant S. aureus (MRSA) to clindamycin via enhancing the affinity between clindamycin and penicillin-binding proteins. Simultaneously, the acidified environment is obviously alleviated by effective infection control, and this in turn enhances the expression of osteogenesis-related proteins, such as alkaline phosphatase (ALP), RUNX family transcription factor 2 (RUNX2), osteocalcin (OCN), and osteopontin (OPN), which also boosts cell competition against bacteria and eventual osteogenesis.
Due to the bacterial resistance to current antibiotics, supramolecular short peptide nanomaterials, such as dipeptides, cyclic peptides, amphiphilic peptides, and self-assembling peptides, have recently gained interest for their potential to combat pathogenic microorganisms [148]. If combined with osteogenic materials, they can provide a promising strategy for simultaneously improving osseointegration and infection prevention. The researchers fabricate a multifunctional PEEK that loads with Lithium ion (Li+) and mussel-inspired antimicrobial peptide on its surface for both ideal implant-bone interface osseointegration and anti-infection effect [149]. The mussel-inspired antimicrobial peptide exhibited outstanding antibacterial properties via penetrating the bacterial cell wall and irreversibly damaging to bacterial membrane, resulting in protein leakage and destruction of adenosine triphosphate synthesis. In addition, the 3D porous structure of PEEK attracts the adhesion and proliferation of BMSCs, and the release of Li+ improves the osteogenic differentiation and osseointegration of stem cells via Wnt/β-catenin signaling. Therefore, the antimicrobial peptides or engineered chimeric peptides successfully combined with osteogenic implants also provide a potentially effective therapeutic way to bacterial drug resistance in clinical practice.
3.1.2. Physical bactericidal capacity and osteogenesis
As above the micro/nanostructures have been proven to promote osseointegration by promoting exosome biogenesis and extracellular secretion [126], however, utilizing its property of physically puncturing bacterial membranes can be regarded as a good strategy for antimicrobial therapy. Due to the negligible side effects of physical bactericidal strategies, Mo et al. demonstrate that argon plasma-treated PEEK surfaces with tilted and vertical nanolamellae penetrate and stretch bacteria, leading to their destruction, with tilted structures being more favourable for peri-implant bone regeneration [150]. In addition, Ye et al. confirmed that the top sharpness and shorter Al2O3-wrapped nanorod patterned array can induce mechano-puncture of S. aureus to achieve the effect of sterilization (Fig. 8a and b) [151]. Fortunately, these sharp nanorods do not damage the viability of BMSCs, which is ascribed to the role of the lower Young's modulus and 3D architecture of the ECM of stem cells [152,153], thereby, simultaneously exhibit excellent osseointegration in bacteria-infected rat tibias models. However, due to the different membrane sensitivities of different bacteria to the nanotopography, the physical puncture is not always effective in preventing implant-associated infection [154,155]. Hence, physical sterilization alone does not seem to be a universal way to alleviate the problem of infection, and it is necessary to combine it with other more effective sterilization strategies for achieving higher synergism between anti-infection and bone-promoting. The antibacterial activity of zinc oxide (ZnO) NPs is increasingly being investigated since it induces intracellular ROS production and mitochondrial dysfunction, which can lead to bacterial death and/or inhibition [156]. On the surface of Ti implants, biofunctionalized ZnO/PDA/arginine-glycine-aspartic acid-cysteine nanorod arrays are prepared for balancing the bacteria-osteoblast race [157]. The special nanorods arrays show the capability to selectively physically puncture bacteria, enhance cytocompatibility, and mitigate cell toxicity through the binding of PDA with Zn2+ to scavenge ROS, which also exhibit excellent ability of infection prevention and osseointegration. In addition, the released Zn2+ can inflow into bacteria through Zn importer and enhance intracellular ROS levels via Fenton-like reactions to achieve a bactericidal effect [158]. The rapid degradation and cytotoxicity of ZnO nanorods limit their biomedical applications, but converting them into stable zinc phosphate (Zn3(PO4)2) via hydrothermal treatment can mitigate these issues while maintaining antibacterial properties [159]. This optimized ZnO/Zn3(PO4)2 hybrid nanostructures exhibits potent antibacterial activity by mechanically disrupting bacterial membranes and concurrently enhances osseointegration in a rabbit model of femoral infection (Fig. 8c).
Fig. 8.
Mechanisms of physical bactericidal capacity and osteogenesis by nanorods. (a) Schematic illustration of the antibacterial mechanisms of nanorods with different top sharpness, showing nanorods with flat top to deform cell envelop appearing bacteriostatic, while nanorods with sharp top to penetrate into S. aureus appearing bactericidal. (b) TEM images of ultrafine structures of S. aureus cultured on Al2O3@ZNRes and Al2O3@ZNR1 for 30 min as well as on Al2O3@ZNRl for 2 h (Blue arrow: electron-lucent region, indicating the leakage of intracellular proteins of bacteria) [151]. Copyright 2022, Elsevier Ltd. (c) Schematic illustration of the fabrication procedures for ZnO/Zn3(PO4)2 hybrid nanostructure (Ti-ZnP2) from ZnO nanorods (Ti-ZnO) to achieve a balance between the anti-bacterial and pro-osteogenic properties [159]. Copyright 2024, Wiley-VCH Verlag.
The advantages of antibacterial coatings prepared by surface modification are obvious, but there are still shortcomings, such as a relatively complex coating process, poor adhesion between the coating and the substrate, easy wear of the coating, and poor long-term antibacterial performance [25,29,160]. In view of the infection problems caused by implanting Ti alloys and the lack of antibacterial coatings, researchers began to try to develop new antibacterial alloy implants with antibacterial functions by adjusting the alloy composition. Meanwhile, the emergence of novel alloy-based biomaterials also displays the strong antibacterial properties and excellent osteogenesis for bone tissue regeneration and repair. For example, Qu et al. designed the biodegradable Zn-Ag alloy implants that possessed remarkable effects of antibacterial, anti-osteolysis, and internal fixation for fractures [161]. Only sporadic bacterial adhesion is observed on the surface of the Zn-Ag alloy and the potential mechanism may be suppressed by the bacterial adhesion colonization genes including Alte and Fbe. What's more, the related gene expression of biofilm formation, autolysis-related pathways, and antibiotic resistance pathways are respectively down-regulated, demonstrating effective antibacterial performance under the release of Zn and Ag ions around the implant during degradation. Compared with the Ti6Al4V alloy implant, the inflammatory response that plays an important role in osteoclastic differentiation is not an obvious change during the degradation of Zn-Ag alloy, which displays reinforced resistance to osteolysis and osseointegration advantages. The successful design of new type of alloys can make up for the long-term deficiency of traditional antibacterial drug treatment method and the generation of drug-resistant bacteria, which also provides a new strategy for advanced smart antibacterial implants.
The bacterial resistance and side-effects of antibiotics remain significant challenges for clinicians treating post-implant infections in clinical practice. It is quite essential to develop safe strategies for eliminating germs without using antibiotics, preventing bacterial infections, and concurrently improving osseointegration to deal with the side effects of antibiotics. During respiration and metabolism, bacteria transfer endogenous electrons to extracellular electron acceptors via the cell envelope c-type cytochromes, whereas in cells, electron transfer occurs within the inner membrane of the mitochondria to produce energy in the form of adenosine triphosphate [162,163]. Due to the structure difference between cells and bacteria in terms of transferring electrons, it also provides new therapeutic strategies for implant-associated infection. Fortunately, the HA/MoS2 coated metallic implants are successfully constructed to interfere with energy metabolism between BMSCs and bacteria (Fig. 9a) [164]. With electrons transfer from the adhered bacteria to the coating surface, the metabolism pathway of S. aureus is altered from aerobic to anaerobic respiration, which induces the disruption of redox balance and then bacterial death. Meanwhile, the coating also promotes the osteoblastic differentiation of BMSCs by altering the potentials of the cell membrane and mitochondrial membrane.
Fig. 9.
Strategies for anti-infection and promotion of osseointegration mediated by biomimetic electrical signals. (a) Electrons extracting from bacteria to HA/MoS2-Ti6Al4V (Ti6) lead to the metabolism pathway changes (left), and the potential of HA/MoS2-Ti6 leads to the osteogenic differentiation of MSCs due to upregulated Ca2+ level (right) [164]. Copyright 2021, Springer Nature. (b) Energy harvesting points of the human body and the structure of triboelectric nanogenerator (TENG) for energizing implant with anti-biofilm and osteogenesis promotion activity. (c) Surface microstructure of the friction layers and general appearance of TENG. (d) Assumed principle of biofilm formation inhibition and promotion of osteogenic differentiation [167]. Copyright 2020, Elsevier.
As mentioned earlier, the positive charge is conducive to the destruction of the bacterial cell wall and cell membrane, similarly, the implant surface with steady and long-term negative charge could effectively inhibit bacterial adhesion and biofilm formation by the electrostatic repulsive force due to the bacterial surface also endowing negative charge [165,166]. Shi et al. innovatively present a self-powered triboelectric nanogenerator to enhance the antimicrobial and osteogenesis properties of implants, which can harvest and transfer mechanical energy from daily human exercise to electrical energy for producing and accumulating numerous negative charge on the surface of anodized Ti implant (Fig. 9b–d) [167]. The constant generation of electric charge on the surface of implants not only reduces the bacterial adhesion but also disrupts the formation of biofilms through synergizing with electrolysis products such as oxidizing radicals. For osteoblasts, the negatively charged biomaterial surfaces can remarkably promote preosteoblast adhesion, proliferation, and differentiation, which may be ascribed to the slightly alkaline environment caused by the increasing pH level around the cathode [168]. In addition, the charged implant surfaces can also induce mineralization from media with mineral ions and recruit the osteogenesis-related cells to accelerate bone healing, that is, the negative potential surface (or film) utilizes galvanotaxis of BMSCs to recruit cells and the positive one attracts electronegative fibronectin to promote cell adhesion [169,170]. These strategies further demonstrates that the biomimetic electrical signals endow the implant with excellent tissue healing function.
3.1.3. External stimuli-mediated bactericidal capacity and osteogenesis
Nowadays, the application of photodynamic therapy and photothermal therapy under NIR light, even the application of electroluminescent material, are extremely enriched antimicrobial treatment methods [171,172]. For example, some researchers have engineered multifarious biomaterials, such as TiO2 nanorod arrays [155], PDA-BP nanosheets/ZnO nanowires [173], black urchin-like defective TiO2/Ag3PO4 nanoparticles [174], among others, and combined with photoresponsive strategy to effectively eradicate biofilms and greatly improve their antibacterial capabilities. Simultaneously, NIR light-mediated mild heat stimulation promotes photothermal osteogenesis, as the proposed hyperthermia (40–42 °C) creates conditions favourable to bone regeneration [175]. Wu et al. devised a ZnL2-BPs@HA scaffold incorporating BPs and a zinc sulfonate ligand (ZnL2), showing the sequential photothermal mediation. This scaffold endows the capability to leverage photothermal therapy for achieving antibacterial efficacy at temperatures below 50 °C in the initial phase, and the promotion of osteogenesis at temperatures ranging from 40 to 42 °C (Fig. 10a) [176]. The detailed antibacterial mechanism involves the synergistic effects of hyperthermia and the positively charged ZnL2-BPs, leading to an augment in intracellular adenosine triphosphate (ATP) and ROS levels in bacteria. This synergistic action, along with the binding of bacteria with negative charges, induces irreversible damage to the bacterial membrane, resulting in protein leakage. Simultaneously, the modified scaffolds contribute to enhanced osteogenesis-related gene expressions of BMSCs through controlled photothermal stimulation and combining the releasing Zn2+ and PO43−, achieving effective repair of bone defects (Fig. 10b and c). Similarly, the ferric oxyhydroxide (FeOOH) nanosheets are used for PEO-coated Mg-based implants, which demonstrates superior photocatalytic/photothermal and antibacterial effects under simulated sunlight and NIR light [177]. The FeOOH films also effectively reduce the corrosion and release of Mg and Fe ions in the Mg implants, as a result, cellular activities such as adhesion, spreading, proliferation, and osteogenic differentiation are considerably increased in vitro, while osteogenesis is further accelerated in vivo. Due to the synergistic effects of multiple therapeutic modalities, combining photothermal therapy with other strategies, such as ultrasound or antibacterial drugs, can achieve better therapeutic outcomes than a single therapeutic modality [178,179]. For example, Su et al. have developed an oxygen deficiency on a Ti implant through sulfur-doping (Ti-S-TiO2-x), endowing the implants with excellent sonodynamic and photothermal abilities [180]. The special implant is processed by near-infrared light and ultrasound, which generates a synergistic antibacterial effect of heat and ROS, and attains an antibacterial efficiency of 99.995 % against S. aureus without introducing any antibacterial coating. In the meantime, the inhibition of implant osseointegration during infection is alleviated and new bone formation is also accelerated.
Fig. 10.
Mechanisms of near-infrared light-mediated bactericidal and osteogenic synergistic effects. (a) Schematic illustration of sequential regulation of the antibacterial and osteogenic ability of zinc sulfonate ligand (ZnL2)-BPs@HA upon mild NIR irradiation. (b) SEM images of the bacteria on (ZnL2)-BPs@HA scaffolds with/without NIR irradiation (Light-/Light+, scale bar = 400 nm). S. aureus shows a golden color, and E. coli is cyan. (c) Micro-CT images of the bone tissues surrounding the (ZnL2)-BPs@HA implants with/without both irradiation I and irradiation II [176]. Copyright 2021, American Chemical Society. (d) Schematic diagram of biofilm eradication (NIR irradiation first and incubation) and biofilm inhibition (incubation first followed by NIR irradiation). (ONOO-: Peroxynitrite) (e) Schematic diagram of the mechanism of promoted bone formation through M1 polarization of macrophages [184]. Copyright 2020, American Chemical Society.
In addition, NIR laser-mediated nitric oxide (NO)-releasing materials have emerged as a promising option for anti-infective treatment, as the released NO can react with superoxides to form peroxynitrite (•ONOO−), which induces oxidative DNA damage and disrupts electron transport in the bacterial respiratory chain, thereby affecting bacterial homeostasis [[181], [182], [183]]. For example, the hydrophilic and adhering hydrogel of poly(vinyl alcohol) is decorated by chitosan, PDA, and S-nitrosuccinic acid, which are immobilized on the surface of the red phosphorus nanofilm modified Ti implants [184]. As shown in Fig. 10d and e, abundant NO was released and •O2− was generated by the special coating under 808 nm NIR irradiation, resulting in the production of •ONOO- to achieve bacteria inhibition and elimination effects. Further research show that photothermal effects could cause direct damage and eradicate the biofilm. Simultaneously, the released NO can promote bone formation and regulate tissue inflammatory cytokine levels, such as TNF-α, IL-6, and interleukin-10 (IL-10), etc., via the M1 polarization of the macrophages. In the same way, S-nitrosoglutathione (GSNO), a nitric oxide donor, is integrated into mesoporous dopamine (MPDA) nanoparticles to surface-modified titanium implants, which triggers a substantial amount of NO to be released quickly by the thermal effect of MPDA when exposed to NIR light [185]. In addition, without NIR light, a spontaneous and gradual release of NO from Ti-MPDA@GSNO substrates can also improve the cytocompatibility and osseointegration by promoting the proliferation and differentiation of osteoblasts. These comprehensive antimicrobial regimens provide new strategies for the design of novel antibacterial and bone-regeneration materials.
3.1.4. Internal microenvironment stimuli-mediated bactericidal capacity and osteogenesis
Bacterial biofilm formation has been proposed as the primary cause of implant infection, which will generate an acidic microenvironment surrounding the artificial implant via the by-products of acidic substances such as acetic and lactic acid during bacterial carbohydrate metabolism [186]. The extracellular polymeric substances of biofilm prevents the penetration of antimicrobials, so surface-modified implants with pH-sensitive biomaterials could be a novel and precise strategy for antimicrobial therapy [187,188]. Shen et al. fabricated a hybrid Mg/Zn-metal organic framework (Mg/Zn-MOF74) coating on alkali-heat treated titanium surfaces that were quickly degraded around the acidic microenvironment [7]. With the alkaline environment created by the degradation products, such as 2, 5-dihydroxyterephthalic acid, Zn2+, and Mg2+, the smart coating showed strong antibacterial ability against both the E. coli and S. aureus and up-regulated the levels of ALP activity, mineralization, and osteogenic gene expression. Other researchers found that a pH-responsive copolymer quaternary ammonium salts-co-methacrylic acid coating on the surface of Ti metal could change from negative charge to positive charge in the acidic microenvironment induced by the infected bacteria (Fig. 11a) [189]. More importantly, the positive charge has been shown to kill pathogenic bacteria by electrostatically attracting the negative charge of the bacterial cell wall and destroying the cell membrane [190]. Therefore, this self-adaptive antibacterial coating can trigger excellent bactericide effects by releasing a positive charge during infection while shielding the positive charge and promoting the adhesion, proliferation, and osteogenic differentiation of BMSCs without infection. Furthermore, Wang et al. also manufactured the 3D-printed bioactive glass scaffold loaded with highly active singleatomic iron catalysts (FeSAC), which displays efficient osteosarcoma ablation, bacterial sterilization, and subsequent osteogenesis during comprehensive osteosarcoma treatment [191]. Under the microenvironment specific to osteosarcoma, the FeSAC produced toxic hydroxyl radicals (•OH) by Fenton catalytic activity, which could achieve the destruction of the osteosarcoma and bacteria through synergizing with the photonic hyperthermia effect mediated by NIR laser. Moreover, osteoinductivity and osteogenesis performances are also remarkably reinforced due to the BMSCs attachment and proliferation induced by the amorphous carbon substrate in FeSAC and enhanced surface roughness.
Fig. 11.
Mechanisms of pH-responsive coatings for antibacterial and osteogenic capacities in infectious microenvironments. (a) Schematic illustration of the self-adaptive antibacterial Ti-based implant with pH-sensitive coating with the dual functions of antibacterial and promoted osseointegration. (PQA: Poly(quaternary ammonium salts-co-methacrylic acid)) [189]. Copyright 2022, Elsevier. (b) Schematic diagram of combining PTT and quorum-sensing-inhibition strategy for improving osseointegration and treating biofilm-associated bacterial infection of Ti-based implant [193]. Copyright 2022, Elsevier.
As mentioned above, the photodynamic and photothermal therapy strategies based on NIR has been widely used in the treatment or removal of biofilm infection via local high temperatures. However, it is unable to completely remove bacterial biofilm infection by utilizing photothermal technology alone because of strong resistance and uneven heat distribution inside the membrane [184,192]. Combined photodynamic therapy with an acidic environment responsive to treating biofilm-associated infection and improving osseointegration could be a precise and efficient strategy. Hu et al. constructed a novel MPDA-LUT@CaP nanosystem on the surface Ti-based implant, which consists of the CaP shell-coated MPDA nanoparticles loaded with luteolin (LUT, a quorum sensing inhibitor) (Fig. 11b) [193]. Interestingly, the CaP shell was rapidly degraded and released LUT, Ca2+, and PO43− from the surface of the Ti implant under the triggering of the acidic environment induced by the bacterial biofilm infection. The thermotherapy mediated by the photothermal conversion action of MPDA under NIR irradiation can effectively inhibit and destroy bacterial membranes and biofilms through synergizing with luteolin. Meanwhile, the release of Ca2+ and PO43− from the surface of the implant accelerates the growth of osteoblasts and the process of bone tissue regeneration. We can thus combine the alterations in the microenvironment around the internal implant with the external photothermal effects to more accurately achieve the therapeutic effects of antibacterial ability and osteogenesis.
Overall, despite the variation in approaches to diverse biomaterials, notable enhancement is observed in the management of biofilm-associated bacterial infections, osteogenic differentiation, and osseointegration performances. Currently, systemic or local antibiotic therapies remain the mainstream treatment for implant-related infections. For instance, the application of vancomycin-loaded bone cement in revision surgeries for periprosthetic joint infections can be regarded as a preliminary clinical trial of functionalized biomaterials, laying the foundation for the development and clinical translation of novel antibacterial biomaterials [194]. However, challenges remain regarding the mechanical properties and long-term stability of novel composite materials, which may impact their durability and clinical applicability. Additionally, the biosafety and potential long-term toxicity of these materials require further investigation to ensure their applicability for synergistic antibacterial properties and bone tissue regeneration in future clinical applications.
3.2. Synergistic interaction between osteogenesis and immunomodulation
Osteoimmunology has revealed the interactions between the immune and skeletal systems, and multifarious cytokines produced by immune cells that can regulate the bone-marrow microenvironment to achieve the dynamic balance of bone formation and resorption [68]. The implant materials are “foreign” as seen by the immune system, and can be phagocytosed or encapsulated by immune cells, which further stimulates the production of large amounts of inflammatory cytokines after implantation [195]. Additionally, the corrosion of metallic implants in biological contexts presents an insurmountable problem for clinical research since it releases metal ions into tissues and body fluids, which tends to stimulate the immune system and bone metabolism and lead to implant failure [196]. To address corrosion-related issues, surface modification, alloying, and deposition of multifarious coatings or thin films on the implants have been proposed as the solution to this challenge [197,198]. The surface modification implants provide better abilities for anti-corrosion and cell-attachment of the metallic substrates [199,200], but the chronic inflammation caused by the imbalance of the immune microenvironment cannot be well resolved and eventually transitions to poor osseointegration. Nowadays, immunotherapy is increasingly used in the treatment of various diseases, such as osteoporosis, bone infection, especially cancer, which can trigger the immune response to achieve excellent therapeutic effects with minimum side effects [201,202]. Accordingly, the concept of osteoimmunomodulation is proposed due to the recognition of the immune response during biomaterial-mediated osteogenesis, which also provides a new promising strategy for developing smart biomaterials for the healing process of bone tissue defect [203].
3.2.1. The mechanism of osteoimmunology
The highly complex immune system acts directly on the skeletal system through the T cells, B cells, and macrophages, or indirectly through the secretion of transcription factors, cytokines, and their receptors. Meanwhile, the alterations in the immune microenvironment elicited by bone biomaterials have been shown to affect bone formation and differentiation of BMSCs, thereby affecting the outcome of bone regeneration [203]. Various immune cells, especially the macrophages, play a significant role in immune defence and osteogenesis, which actively engage in inflammatory response to achieve advanced tissue restoration effects [204,205]. During bone reconstruction, macrophages and their precursor (monocytes) not only phagocytize necrotic tissue fragments, remove implant-wearing debris, and regulate bone resorption. They also secrete a variety of cytokines and growth factors, directly instructing osteogenic differentiation of osteoprogenitor cells and bone matrix remodelling [206]. The macrophage can be categorized into either type M1 or type M2 depending on where it is in its life cycle and the role it performs. In simple terms, M1 macrophages are known to reinforce the inflammatory response and regulate the osteoclastic process by producing these proinflammatory cytokines such as TNF-α, IL-1β, IL-6, etc., nevertheless, M2 macrophages alleviate inflammation responses and ameliorate tissue repair by secreting anti-inflammatory cytokines, such as IL-10 and TGF-β, to ultimately induce bone formation [207,208]. The presence of M1 macrophages over an extended period of time may result in fibrotic scar tissue formation and tissue harm [209]. Accordingly, to achieve better bone tissue repair and osteointegration surrounding the implants, macrophages must shift from proinflammatory (M1) to anti-inflammatory (M2) phenotypes that are conducive for the bone immune milieu.
Numerous researchers are trying their best to design inert biomaterials to reduce the immune responses caused by implants in the past times. Regrettably, this strategy seem not to work well and even causes implant failure owing to insufficient blood supply [210]. Nowadays, there is a growing acknowledgement of the benefits associated with the immune response in tissue remodelling. The osteoimmune microenvironment is influenced by both innate and adaptive immunity and plays a pivotal role in the osteogenic process. For example, the anti-inflammatory M2 polarization of macrophages has been validated as a means to modulate the inflammatory response and acts as an osteogenic mediator, thereby enhancing osteogenesis [33,211]. Similarly, B lymphocytes can release interferon-γ and OPG to suppress the formation of osteoclasts, thus preventing bone resorption [212]. However, the activated T lymphocytes can secret RANKL to stimulate the formation of osteoclasts and thus enhance the occurrence of osteolysis [213]. Nowadays, many novel bioactive implants with positive immunomodulatory functions are exploited to improve the host-implant interaction via utilizing the immune system [214]. As the innovative strategies to enhance bone repair, the development of delivery systems with growth factors, peptides, small molecules drugs, and even cytokines targeting the resident immune cells could reshape inflammatory and immunity processes to achieve better osteoinduction and osteogenesis [215,216]. Incorporating immunomodulatory molecules or specific ligands into biomaterials represents a promising way for stimulating and accelerating bone healing. For example, sitagliptin, a medication inducing macrophage polarization to the M2 phenotype, has been integrated into injectable macroporous silk gel scaffolds. This integration facilitates local sustained release and maintains an optimal drug concentration at the bone-implant interface [217]. In comparison to oral sitagliptin administration, the silk/sitagliptin gel scaffolds remarkably induced effective recruitment of M2 macrophages to the implants and ameliorate the inflammatory responses under diabetic conditions, thus potentiating bone tissue regeneration and osseointegration.
Furthermore, during bone development and repair, BMPs play a crucial role in stimulating bone formation by interacting with BMP receptors and activating intracellular signal transduction. Amng them, BMP-2 shows positive immunoregulatory effects, including macrophage recruitment and polarization through the activation of the pSmad1/5/8 signaling pathway [218]. With the advent of 3D bioprinting technologies, the combination of cellular or molecular protein and implant materials has become a reality [219], which greatly expands the research scope of implants and better promotes bone tissue repair. The 3D-bioprinted scaffolds, constructed by the composite bio-inks with gelatin, GelMA, poly (ethylene glycol) acrylate, and BMP-4-loaded mesoporous silica nanoparticles, facilitate the delivery and sustainable release of BMP-4 for a long-term immunomodulatory effect [220]. The released BMP-4 not only induces the polarization of RAW264.7 towards the M2 phenotype but also enhances the osteogenic differentiation of BMSCs, synergizing with the secretion of BMP-2 by M2 type macrophages to further boost bone tissue regeneration. Thus, adapting and harnessing the immune response emerge as a crucial factor in designing smart implants to promote bone tissue regeneration.
3.2.2. Inherent immunomodulatory properties of biomaterials
As shown above, the topography and morphological properties of implant surfaces are essential to facilitate osteogenic differentiation of stem cells and osteointegration in vivo. Similarly, researchers found that surface topography cues play a critical role in mediating macrophage polarization by modulating cell shape, thereby inducing macrophages to create a favourable microenvironment for osteogenesis [221,222]. Zhu et al. successfully fabricated the 90-nm honeycomb-like TiO2 structures on titanium substrates, which could significantly promote osteogenic differentiation of BMSCs in vitro and subsequent osteointegration in vivo by ameliorating the immune microenvironment [33]. The TiO2 honeycombs endow macrophages with the ability to overcome the surface spatial confinement and facilitate more filopodia formation, resulting in M2 macrophage polarization. The specific mechanism underlying this phenomenon is ascribed to the activation of the Rho family of GTPases (RhoA, Rac1, and CDC42), which upregulates the RhoA/ROCK signaling pathway (Fig. 12a and b). Due to the suitable immune microenvironment provided by TiO2 honeycomb-induced M2 macrophages and the secretion of cytokines IL-10, IL-4, and BMP-2, the osteogenic differentiation of BMSCs and final osteogenesis and osteointegration are significantly promoted. Similarly, some researchers also demonstrate that Sr is incorporated into the Na2TiO3 nanorods arrays by the substitution of Na + using hydrothermal treatment, which ameliorates osteogenic immune microenvironment via accelerating macrophage polarization towards M2 phenotype [223]. In addition, the released Sr2+ enhances the expression of integrin subunits to promote the formation of focal adhesion and also enhances cell adhesion and filopodia formation of macrophages through synergizing with the rough nanorod array surface. More importantly, researchers further report that the Sr2+-incorporated implants can accelerate new bone formation and osseointegration in diabetes via regulating macrophage polarization and Wnt5a signaling [224]. Thus, the Sr2+ and other osteogenic-related ions incorporated into bioactive implants can directly enhance the osteogenic function of osteoblasts and exhibit the best performance in osseointegration by changing implants’ immunomodulatory capability.
Fig. 12.
Effects of surface nanostructures and electrical potential on macrophage polarization and osteogenic differentiation. (a) Cell morphologies of RAW 264.7 macrophages on the surfaces of the bare Ti and Ti with 90-nm (diameter of polystyrene spheres) TiO2 honeycomb-like structures. (HC: honeycomb) (b) Scheme illustration of the mechanism of macrophage polarization on the special honeycomb nanostructures [33]. Copyright 2021, American Association for the Advancement of Science. (c) Schematic diagram of cellular osteogenic differentiation on different potential of polyvinylidene fluoride trifluoroethylene (P(VDF-TrFE) films [237]. Copyright 2018, Elsevier Ltd.
In addition to the immunomodulatory effects of the modified micro/nanostructures on the implant surface, osteogenesis-related biomaterials would also have outstanding efficiency for immune microenvironment modulation. For instance, calcium silicate bioceramics, as a class of biomaterials for hard tissue repair, have been demonstrated to significantly promote osteogenesis and bone tissue regeneration by regulating BMSCs [116,225]. However, Zhou et al. reported that the calcium silicate bioceramics possess excellent osteoimmunomodulatory properties to stimulate osteogenesis via the M2 phenotypic transformation of macrophages and the upregulation of macrophage-derived oncostatin M (OSM) [226]. Under the calcium silicate extract simulation, the expression of M2 macrophage surface markers such as CD206, IL-10, and TGF-β, are significantly elevated, which causes better host-to-material anti-inflammatory effects. In addition, the macrophage-secreted OSM promotes osteogenic differentiation of BMSCs by activating the ERK1/2 and JAK3 pathways, being conducive to osteoinduction and osteogenesis. Similarly, as the most commonly used auxiliary material in clinical orthopaedic surgery, tricalcium silicate-based cements also attract more attention in terms of their roles in bone regeneration [227]. With the stimulation of tricalcium silicate-based cements, the macrophages can be polarized towards the M2 phenotype on the material-tissue interface, which is conducive to participation in bone tissue healing via the secretion of cytokines including BMP2, TGF- β1, and vascular endothelial growth factor (VEGF). Recently, research has shown the polarization state of macrophages is extremely sensitive to the physicochemical properties of biomaterials [228]. Due to this recognition, we should actively utilize the ability of the new smart implants to modulate the immune behaviour in vivo and promote M2 polarization of macrophages so as to efficiently resolve inflammation and better achieve the healing and reconstruction of bone tissue.
3.2.3. Endogenous biomimetic microenvironment for osteoimmunomodulation
With deeper understanding of the native tissue microenvironment, researchers have found that the biomimetic endogenous physicochemical microenvironment created by smart implants may directly regulate M1/M2 macrophage polarization [229,230], which would achieve advanced osteogenesis effects compared with the biomaterial itself. The changes in the electrical microenvironment around implants have been found to play a crucial role in immune modulation, presumably regulating macrophage recruitment, polarization, and cytokine production [170,231]. Thus, the electrical signaling could offer a viable method for modulating osteoimmunomodulatory effects in order to enhance osteogenesis. For example, Dai et al. demonstrated that a ferroelectric BaTiO3/poly (vinylidene fluoridetrifluoroethylene) nanocomposite membrane could produce the biomimetic negative electrical microenvironment, which exhibited excellent osteoimmunomodulatory effects to promote osteogenesis [232]. Interestingly, the negatively charged nanocomposite membrane is implanted, covering the negatively charged bone wall of the defect area, and can form an enclosed endogenous electrical microenvironment to mimic natural bone ECM. By inhibiting the expression of AKT2 and IRF5 within the PI3K/AKT signaling pathway, this induces macrophages to the M2 phenotype with lower IL-6 secretion, especially in hyperglycemic conditions. As we know diabetes mellitus triggers excessive inflammatory activation of macrophages and hinders the transformation of macrophages towards an anti-inflammatory state during bone healing [36,233]. Therefore, given the mechanism of action of the novel nanocomposite membrane, the electrical microenvironment and changes in electrical signaling around the implant provide a promising strategy for facilitating bone regeneration, particularly in the diabetes microenvironment.
Physiological electrical signals have been proven in biological research to maintain the morphology, intracellular energy metabolism, and information transfer of living cells [234,235]. Nowadays, the bioelectric signals effectively polarize macrophages toward the M2 phenotype by inhibiting the inflammatory MAPK signaling cascade and activating oxidative phosphorylation to modulate the immune microenvironment [236]. For instance, research has shown that the surface potential of biomaterials can dramatically influence cellular osteogenic differentiation, and actually reach the optimum osteogenic differentiation level of MC3T3-E1 cells on the ferroelectric polyvinylidene fluoride trifluoroethylene film with 391 mV, which is attributed to the binding state of the adsorbed fibronectin with integrin (Fig. 12c) [237]. Other researchers indicate that the PDA coatings on the implant surface are conducive to decreasing its surface potential, which subsequently polarizes macrophages towards the M2 phenotype via inhibiting the PI3K/Akt/mTOR signaling pathway [238]. Simultaneously, under the lower surface potential, the expression of adhesion-related genes is upregulated by the FAK pathway and produce a repulsive force against bone marrow-derived macrophages (BMDMs) for inhibiting osteoclast formation. The excellent antibacterial effect of Ag-containing biomaterials has been widely demonstrated, however, implants doped with the appropriate amount of Ag also can effectively regulate immune response and promote new bone formation [239]. It is also discovered that Ag-doped Ti implants can produce the micro-galvanic effect, which could boost osseointegration by regulating the immune response of macrophages [240]. The micro-galvanic impact is amplified as the quantity of Ag increased, and macrophage voltage-gated Ca channels are receptive to opening and facilitating Ca2+ influx. Although the Ca2+-dependent PKC-NF-B signaling pathway polarizes macrophages towards the M1 phenotype, the production and release of Ca-dependent prostaglandin E2 (PGE2), which dominates the immunological response induced by implants, and contributes to the enhancement of the M2 phenotype polarization. Meanwhile, the presence of BMSCs may restrict Ca2+ influx and stimulate the production of PGE2, to promote M2 macrophage polarization, which eventually boosts osteogenesis and implants osseointegration actions.
In addition to the electrical microenvironment, mimicking the endogenous magnetoelectric microenvironment is also an efficacious strategy for biomaterial-mediated tissue repair and remodelling [241]. Liu et al. engineer the CoFe2O4/poly(vinylidene fluoridetrifluoroethylene) magnetoelectric nanocomposite membrane for precisely controlling bone regeneration in the host bone-implant interface [242]. With the stimulation of the appropriate magnetoelectric microenvironment, the magnetoelectric membranes can directly activate the initial immune response and subsequently accelerate polarization of anti-inflammatory M2 phenotype possibly through the RGD-integrin binding induced PI3K/Akt signaling activation. Simultaneously, the osteogenic differentiation level of BMSCs is significantly elevated because of the exposure of RGD (a key cell-adhesion peptide) site and maturation of focal adhesion. Accordingly, the surface potential of an implant is a vital factor that influences the immune microenvironment and cellular behaviour. Variations in surface potential can significantly impact protein adsorption, BMSCs adhesion, and immune cell activation, thereby affecting the immune microenvironment and osteointegration between the implant and bone tissue. Therefore, careful consideration and strategic manipulation of the surface potential are essential in the design and development of smart implants to ensure their long-term functionality and osteointegration within the host tissue.
Recent studies have disclosed that the interactions between the hemostatic and immune systems effectively induce osteoimmunomodulation to accelerate bone healing, especially in the bone defect area [243]. The innate immune response can be efficiently activated by a blood clot composed of platelets and plasma carrying numerous growth factors, resulting in the recruitment of many different immune cells that are beneficial to bone repair [244]. Fan et al. designed a blood clot gel delivery platform loaded with BMP-2 protein for its local delivery around the bone defect site to accelerate the healing process of bone tissue [245]. The released BMP-2 tends to ameliorate the bone osteoimmunological microenvironment by recruiting more macrophages and regulating their polarization state, which achieves effective anti-inflammatory effect to stimulate osteogenesis. Compared with free BMP-2, the BMP-2-loaded blood clots significantly upregulates the expression of some osteogenic proteins such as Runx2 and Osterix, which may be ascribed to the endogenous factors of the blood clot. More importantly, due to the deep-red colour, blood clots could generate mild localized hyperthermia effects under the 808 nm laser irradiation, effectively regulating the osteoimmunology associated with the healing of bone defects to reinforce bone tissue regeneration. By combining immunomodulatory and photothermal effects, maximizing the osteogenesis-related performance of the novel hematoma biomaterials provides an effective strategy to repair bone defects. Meanwhile. The smart hematoma-based implants enable the creation of innovatively targeted delivery techniques to influence cellular behaviour for a variety of regenerative medicine applications, including regulation of the osteoimmune microenvironment and personalized bone defect regeneration. Taken together, the endogenous physical and chemical conditions can effectively ameliorate the osteoimmunomodulatory environment and promote osteogenesis, which provides a promising and novel therapeutic strategy for bone tissue regeneration.
3.2.4. Sequential osteoimmunomodulation to facilitate osteogenesis
Numerous researches have shown that improving the immune microenvironment by modulating and promoting the polarization of M2 macrophages through biomaterials plays a leading role in the bone repair process. However, the positive effect of M1 macrophages on immune cell recruitment and the clearance of cell debris seems to be ignored, which attenuate the effectiveness and performance of implant materials for bone healing through osteoimmunomodulatory strategies [246]. At the initial stage of implantation, as an exogenous material, bone implant will inevitably cause the host immune response and produce certain physiological inflammatory responses. The osseous regeneration processes start with a transient inflammatory phase, demonstrating the significance of pro-inflammatory macrophages in precise temporal osteoimmune modulation to optimize bone repair and regeneration. Studies report that the M1 polarization of macrophages likely substantially increased BMSCs attachment and migration while also promoting BMSCs with differentiation toward the osteoblast lineage via the COX-2-PGE2 pathway during early osteogenesis [247,248]. Due to this recognition, M1 macrophages predominate during the early stage of inflammation and are actively engaged in bone tissue repair, which can be harnessed to recruit more additional effector cells and precisely induce macrophage transformation towards an anti-inflammatory phenotype in the following time. Currently, a programmed PEEK surface has been designed for immune-mediated osteogenic regulation via the sequential release of IL-10 and dexamethasone [249]. This process initiated an aptly weak inflammatory response, followed by sustained dexamethasone release that creates an optimal immune environment, thereby further enhancing osteogenesis and bone formation. Simultaneously, the immunomodulatory mechanisms and approaches can be used to temporally regulate the phenotypic transformation of macrophages from M1 to M2 polarization in combination with various biomaterial platforms, thereby enhancing bone regeneration and achieving more advanced osteogenic effects.
It is precisely because of the existence of physiological inflammatory response and excessive ROS generation upon implantation, which significance hinders the osteogenesis around the host bone-implant areas. Li et al. created a PDA@HA nanorod-like array on the Ti surface by combining the anti-oxidant PDA with the osteogenic HA, which displays excellent sequential osteoimmune regulation functions (Fig. 13a–c) [250]. The novel implants have immunomodulation and ROS scavenging capabilities under periodic photo-thermal therapy, which manipulates the recruitment and osteogenic differentiation of BMSCs. More importantly, the instantaneous thermal stress can induce pro-inflammatory macrophage polarization by the release of heat shock protein 70, while the following mild photothermal effect (41 ± 1 °C) would incline polarization towards anti-inflammatory phenotype that is ascribed to the activation of the PI3K/Akt signaling pathway. By combining with photothermal effects, the sequential osteoimmune modulation on macrophage polarization can provide a favourable immune microenvironment for achieving advanced bone healing. Due to the NIR can only penetrate a certain depth in biological tissues, magnetic hyperthermia therapy in deep tissues would be a better choice for the treatment of bone defects [251,252]. Nowadays, magnetically responsive biomaterials, such as Fe3O4 NPs, are frequently utilized to alter the interactions between cells and materials [6]. Due to their magnetic hyperthermia effects and ability to promote the osteogenic differentiation of BMSCs under an external magnetic field, these materials show great promise for applications in bone tissue regeneration [253,254]. Huang et al. constructed a magnetically responsive superparamagnetic NPs hydrogel that recruited adequate M1 macrophage infiltration after implantation and then enhanced M2 polarization of macrophages at the middle/late stages of bone healing under the magnetic field conditions (Fig. 13d–f) [255]. Sufficient M1 macrophage infiltration can be prepared for subsequent phagocytosis of debris and recruitment of progenitors. Meanwhile, under suitable magnetic field conditions (280 mT), the nanocomposite hydrogel induces the M1-to-M2 transition of macrophages via the podosome/Rho/ROCK mechanical pathway so as to achieve optimal osteogenic functions in vivo. Therefore, while concentrating on the powerful effect of macrophage M2 polarization on osteogenesis and the immune microenvironment, we cannot ignore the important role of M1 macrophages in the early stage of inflammation. The timely macrophage polarization and sequential osteoimmune regulation strategies would precisely control the inflammatory progression and produce more advanced osteogenesis and osseointegration effects.
Fig. 13.
Sequential osteoimmunomodulation strategies to facilitate osteogenesis through external stimuli-mediated macrophage polarization. (a) Schematic illustration of the periodic photo-thermal treatment (PTT) process in vitro for evaluation of the phenotypic transformation of macrophages cultured on 2PDA@HA with 2 mg mL−1 DPA. (b) Schematic illustration showing the mechanism of PTT induced macrophage polarization. (c) Gene expressions of the RAW264.7 cultured on the PDA@HA arrayed Ti for 1, 4, and 7 days: anti-inflammatory cytokines CD206 and pro-inflammatory cytokines TNF-α [250]. Copyright 2022, Wiley-VCH GmbH. (d) Schematic illustration of magnetized nanocomposite hydrogels for on-demand immunomodulation via temporally controlled macrophage phenotypic transition in response to a magnetic field. (e) Photographs of cranial samples and the corresponding 3D reconstruction images by micro-CT. The blue circles represent the initial defect areas, diameter = 5 mm. (f) Quantitative analysis of new bone formation at the site of implantation (defect coverage and bone volume) [255]. Copyright 2022, Wiley-VCH GmbH.
The important role of macrophage polarization during the process of bone tissue regeneration seems almost self-evident, moreover, other innate immune cells like neutrophils, dendritic cells, and natural killer cells, etc., have also played indispensable roles in the healing process of bone tissue following the implantation [[256], [257], [258]]. For example, interleukin-8, a chemotactic cytokine for neutrophils, can immediately recruit neutrophils during the physiological inflammatory response around the implanted site, which further functions as initiator to recruit BMSCs and macrophages, and consequently induce rapid endogenous bone repair [259]. Meanwhile, as an important part of temporal immune regulation, the adaptive immune system including regulatory T cells and T cell-derived cytokines, also plays the positive roles in BMSC recruitment and accelerates endochondral ossification following biomaterial implantation [260,261]. Yu et al. have demonstrated that the biomimetic HA nanorods, particularly nanorods with an approximate aspect ratio of 100, significantly increased the percentage of T cells and induced the endogenous production of IL-22 [262]. As shown in Fig. 14a, more importantly, the IL-22 activated the janus kinase 1 - signal transducer and activator of transcription 3 (JAK1-STAT3) signaling pathway in BMSCs to increase the expression of osteogenesis-related genes (Runx2, Osterix, ALP, OCN, and OPN), which demonstrated the greatest potential to stimulate osteogenesis. Thus, combining HA nanorods and different biomaterials is viewed as the supplementation of innate immune regulation, which would ameliorate the adaptive immune microenvironments on osteogenesis and provide a promising approach for bone defect reconstruction.
Fig. 14.
Adaptive immunomodulatory strategies for enhancing osteogenesis. (a) Schematic illustration of the osteogenic activity regulated by underlying immunomodulatory response focusing on the functions of T cells and T cell-derived cytokine (IL-22) of the injectable SA-HA nanorod composites with different aspect ratios of HA (HA-0, HA-30, and HA-100) [262]. Copyright 2022, American Chemical Society. (b) Schematic illustrating the Au nanocages (GNCs)-modified biphasic CaP (BCP) with the immune response characteristics of macrophages and dendritic cells (DCs). The GNCs-modified BCP endows controlled release of IL-4 at the early-stage implantation and dexamethasone (DXMS) at the late stage, facilitating macrophage M2 polarization and inhibiting DCs maturation for promoting materials-mediated new bone formation [264]. Copyright 2020, Springer Singapore.
At the same time, during the occurrence and development of physiological inflammation in a normal body, innate immunity serves as the initial line of defence against various inflammatory responses and provides important cues for adaptive immunity development [263]. Similarly, how to precisely regulate innate and adaptive immune responses to stimulate osteoinduction in the process of bone tissue reconstruction provides a new idea for designing a smart implant in bone tissue engineering. Interestingly, Zhao group successfully engineered a dual-wavelength photosensitive micro/nano-hierarchical scaffold consisting of IL-4 or dexamethasone, which embodied a new paradigm with precisely regulating innate and adaptive immune responses (Fig. 14b) [264]. Under 690 nm NIR light, the IL-4 is released from the innovative scaffold and targets the M2 polarization of macrophages in the early stage of osteogenesis. During the later stage, scaffolds would then release dexamethasone under 808 nm NIR, targeting the immature dendritic cells and inhibiting dendritic cells maturation through synergizing with biphasic CaP stimulation. Other researchers also found that the excessive T cell activation hinders bone tissue regeneration and lowers the biocompatibility of biomaterials, often resulting in foreign body rejection and even implantation failure [265]. As professional antigen-presenting cells, dendritic cells can activate naive T lymphocytes and initiate potent adaptive immune responses. Thus, above biphasic CaP-induced immature dendritic cells indirectly suppress T cell activation, which not only offers an adaptive immunological milieu that favors BMSC osteogenic differentiation but also provides a crucial regulatory hub between innate and adaptive immunity. Although various academics have somewhat different perspectives on the osteogenic function of adaptive immunity [262,265], the sequential immune regulation of bioactive implants may effectively create a desirable immunological milieu of osteogenesis and accelerate bone remodelling. The important hints from the interactions between the smart implants and various immune cells in the osteoinductive process, can bring novel thoughts and strategies for designing a new generation of endogenous bone repair biomaterial and may also drive research into the novel therapeutic approaches of bone tissue defects.
As discussed above, the interactions between bone tissue and the immune system can be facilitated by implantation of a variety of biomaterials. These materials demonstrate the ability to manipulate and regulate the host immune system to promote key regenerative effects, for example, initiating bone tissue repair, lessening inflammatory response, and promoting osteoblast differentiation. However, various immune cells and their factors are the major source of mediators of body inflammatory response [266], and inappropriate or unrestricted activation of immune responses may impair host immunological homeostasis and subsequent osseointegration. Hence, the development of smart bone implants with suitable immunomodulatory response would assist in the resolution of complications in bone healing, the development of more effective and safer therapeutics, and even regenerative engineering techniques.
3.3. Synergistic interaction between osteogenesis and angiogenesis
The role of vasculature in bone and osteochondral development, growth, and repair has been well documented. Vascular invasion is required for endochondral ossification and the ossification of cartilage templates in fetal skeletal development and postnatal bone repair [267]. The densely distributed vascular networks can deliver nutrients and oxygen to the cells, while also eliminating metabolic products away from them. Therefore, excellent osteogenic and pro-angiogenesis capabilities also play a critical role in the bone healing process after the bone defect or orthopaedic implantation procedures [268,269]. In recent years, scientists in bone tissue engineering focus increasingly on the impact of various implant biomaterials on angiogenesis during bone regeneration, aiming to elucidate the interconnected mechanisms governing bone regeneration and vascular formation.
3.3.1. Biodegradable implants for angiogenesis and osteogenesis
Bone scaffolds, especially the porous implants, are the most commonly used biomaterials in bone defect repair and treatment because they exhibit effective biomechanical support and provide a foundation for bone ingrowth [270,271]. However, scaffolds with simple supporting ability can hardly meet the needs of bone reconstruction. How to establish a good vascular network surrounding the implant to provide sufficient blood supply for better osseointegration is still certain challenge. By regulating the surface microstructures and doping bioactive factors and elements such as Mg2+ ions and growth factors, the inert implants can endow therapeutically advantages and become more intelligent in clinical practice [272,273]. Meanwhile, the selected elements are mainly the essential factors in normal bone metabolism, which can achieve biomimetic strategies for facilitating osteogenesis and vasculogenesis during bone defect reconstruction and bone fracture healing [274].
Researchers recently discovered that HA bioceramics with micro/nano-hybrid (a mixture of microrods and nanorods) surfaces and Sr2+ doping obviously enhance the bone and endothelial cell spreading and proliferation [275]. Compared with other bioceramics, the nanostructured surface and Sr ions have synergistic effects on osseous regeneration via enhancing gene expression of osteogenic and angiogenic factors, including BMP-2, OPN, VEGF, and ANG-1. In the process of bone reconstruction, early angiogenesis plays an important role in scaffold survival. At the same time, Mg2+ is reported to promote the activity of magnesium transporter subtype 1 (MagT1), which results in Mg2+ influx and then stimulates the VEGF transcription of human umbilical vein endothelial cells (HUVEC) via hypoxia-inducible factor (HIF-1) activation to induce angiogenesis (Fig. 15a–d) [276,277]. For example, the biofunctional Mg-coated porous Ti6Al4V scaffold can stimulate early blood vessel formation as well as osseointegration and osteogenesis around the implant [278]. Aside from the proliferation and adhesion effects on bone cells and HUVECs, the special Mg-coated scaffold can also improve ECM mineralization and ALP activity when compared to the bare Ti6Al4V scaffold. Nevertheless, it remains the challenge for implants to deliver various ions in smart controlled-release manners while minimizing or eliminating the problems of burst release [279,280]. To address the issues of precise ratio-controlled delivery around bone healing sites, Wan et al. recently constructed the hierarchical therapeutic ion-based microspheres with optimized sustained release ratios of Mg2+ and Si4+ [281]. During the initial period of implantation, the mild burst release of Mg2+ facilitates cell recruitment and new vascular formation. Subsequently, the release of Mg2+ and Si4+ would achieve an optimal ratio of 2:1 and remain at a certain level, significantly accelerating bone healing process via enhancing angiogenesis, collagen synthesis, and biomineralization. Due to the controlled release of bioactive ions throughout the persistent bone repair process, this type of smart implant demonstrates complimentary and synergistic capabilities in controlling angiogenesis and osteogenesis. Meanwhile, the controlled approach for the coordinated release of diverse ions can open a new avenue for developing therapeutic ion-based bone scaffolds in bone tissue engineering.
Fig. 15.
Bioactive components-modified smart implants for vascularization and osteogenesis. (a) Schemata of construction of the Mg-enriched 3D culture system for bone regeneration. Mg2+ influx via magnesium transporter subtype 1 (MagT1) mediates the osteogenic differentiation of stem cells. (b) ALP staining of BMSCs treated with or without PD98059. (c) Semiquantitative analysis of ALP activity in BMSCs. Mg2+ promotes osteogenic differentiation through selective activation of the Erk1/2 signaling pathway (depressing specific inhibitor PD98059). (d) Evaluation of neovascularization in nude mice in vivo. Blood perfusion in each group was detected by laser Doppler imaging (LDI) at different time points. Implantation sites are circled. A Mg-enriched environment stimulated neovascularization from days 3–14 [277]. Copyright 2019, Wiley-VCH Verlag. (e) Schematic diagram depicting the detailed mechanisms involved in the improvement of osteogenesis and angiogenesis by miR-1260a derived from BMSC-Fe3O4-SMF-Exos. (SMF: static magnetic field; Exos: Exosomes) [298] Copyright 2021, Springer Nature.
In addition, the development and rise of biodegradable metallic biomaterials have brought about a revolutionary change in implants [282]. With the formation of new tissue, the biodegradable implant continuously dissolves and releases appropriate ions into the neighboring biological fluid and tissue to synergistically promote tissue reconstruction and repair [283]. For example, the Nb2C MXenes nanosheets (NSs) decorated 3D-printed bioactive glass scaffolds (NBGS) have been indicated the capability of angiogenesis and facilitate osseous repair and remodelling to heal large bone defects [284]. As the stent biodegrades, the releasing Nb2C could persistently enhance the expression of angiogenesis-related genes such as VEGF-B and FGF2 to promote better vasculogenesis and migration of blood vessels. The new vessels would transport more oxygen, vitamins, and energy around the bone defect areas for osseous regeneration. Furthermore, as biodegradable metallic materials, Mg-based alloys provide a regulated release of metallic Mg, Ca, and Zn ions, etc. at a rate that facilitates angio-osteogenesis for better bone healing. For example, the novel Mg5Ca1Zn alloys promote rapid bone repair by releasing anabolic metallic ions within the bone microenvironment to encourage blood vessel formation and actively recruit osteoprogenitors around the surrounding tissues [285]. In the same ways, Liu et al. engineered MgSnZnNa alloy presented improved osteogenesis and angiogenesis capacity due to the co-release of Na and Mg ions [286]. As essential elements, Na+ is safer compared with other alloying elements, resulting in advanced upregulation of OPN and OCN gene expression, calcitonin gene-related peptide as well as osteocalcin to promote osteogenesis of osteoblast. Simultaneously, the VEGF level was significantly upregulated in accordance with blood capillary-like structures found in the new bone. The manufacture of new biodegradable metal alloys also provides new options for the supplementation and development of smart implants. And the properties of biodegradation and releasing ions manifested excellent osteogenic and angiogenic abilities and provided new promising approaches for clinical bone defect repair in the future.
3.3.2. Bioactive components-modified implants for vascularization and osteogenesis
The healing process of skeletal system regeneration cannot be solved only by the nature of the biomaterial alone. In order to achieve more intelligence functions for promoting osseointegration and reconstructing related tissues, the novel implants can be co-deposited or modified by biologically active components such as drugs, cell growth factors, and biologics [[287], [288], [289]]. Researchers have recognized that the significance of slow-release bioactive components from implant surfaces in synergistically regulating angiogenesis and osteogenesis [290]. Therefore, bioactive implants can provide a platform for safe and effective delivery in clinical applications that the increased osteogenesis and neovascularization are favourable.
Nowadays, drugs and biologics are used in combination with different implant materials to synergistically promote osteogenesis and related tissue remodelling. Previous results show that type H vessels, the specialized microvascular components are distributed in the metaphysis of bone, can promote the coupling of angiogenesis and osteogenesis via high expression of Endomucin and CD31 [[291], [292], [293]]. This also reveals the role of interactions between type H endothelial cells and osteoblasts in both normal bone injury and defect repair. Recently researches found that the simvastatin (a hypolipidemic drug) loaded onto sulfonated PEEK surface significantly enhances the formation of type H vessels effect [294]. Under the slow steady release of simvastatin, the level of miR-29cb2, a novel coupling factor that regulates osseointegration and angiogenesis, is significantly inhibited and then suppresses the HIF-3α expression, leading to the formation of type H vessels and neonatal bone tissues. Similarly, the pitavastatin is also proved to have the function of synergistic osteogenesis-angiogenesis regulation, which is used to construct the multifunctional multilayer films on the surface of metal implants by layer-by-layer (LbL) assembly method [295]. Meanwhile, the locally released pitavastatin also regulates the paracrine signaling-mediated interaction between BMSCs and endothelial cells, promoting the coupled osteogenesis and angiogenesis and ultimately improving osteogenic reaction at the implant-bone interface.
Recent researches demonstrate that the exosomes derived from different cells can induce fracture healing and osseous regeneration by accelerating angiogenesis in an in vivo and in vitro models [296,297]. For example, Wu et al. constructed a novel type of exosomes derived from BMSCs under the stimulations of magnetic Fe3O4 NPs combined with a static magnetic field [298]. As shown in Fig. 15e, the modified exosomes exhibit excellence in osteogenesis and angiogenesis, which is attributed to the highly enriched miR-1260a inside them. Furthermore, the exosomal miR-1260a also plays a pivotal role in enhancing collaborative osteogenesis and angiogenesis through suppression of histone deacetylase 7 (HDAC7) gene in BMSCs and collagen type IV alpha 2 (COL4A2) gene in HUVECs, respectively. Another study have also shown that the exosomes containing miR-142-3p derived from the regulatory T-cells can be transferred to BMSCs and HUVECs, and then potentiate osteogenesis and angiogenesis [299]. Therefore, the combination of exosome and bone implant materials will provide a significant strategy to promote the treatment of bone defects. Otherwise, the exosomes derived from umbilical MSCs are encapsulated within an injectable hyaluronic acid hydrogel and then filled in the customized 3D nanoHA/poly-ε-caprolactone scaffold, which exhibits accelerated angiogenesis-dependent bone formation [300]. The main reason is that miR-21 overexpresses in the exosomes and suppress the NOTCH1/DLL4 signal pathway while inducing VEGF and HIF-1α expression as an intercellular messenger. To date, it also launches a new era of modularized sEVs therapy in tissue engineering through the “functional design” of EVs components including the lipid bilayer, proteins, and nucleic acids [[301], [302], [303]]. For bone tissue engineering, Tao et al. successfully developed the modularized sEVs with cyclo(Arg-Gly-Asp-d-Phe-Cys) surface functionalization and Zinc Finger E-Box Binding Homeobox 1 (ZEB1) loading, by a coated on TCP scaffold, and then coated on the β-TCP scaffold that achieves remarkable osseointegration effects [304]. In detail, the modularized sEVs target the ECs, BMSCs, and pre-OCs through αvβ3, and increase the levels of ZEB1 levels in the functionalized bioscaffolds. The ZEB1 then enhances the coupling of angiogenesis and osteogenesis via ZEB1-YAP interaction and inhibits OCs maturation by downregulating NFATc1, which endows excellent osseous regeneration, especially in the healing process of diabetic bone defects.
Bioactive components-modified implants are emerging as a promising strategy for improving the synergistic regeneration of angiogenesis and osteogenesis, which is a critical process in bone tissue engineering. Integrating bioactive components onto implant surfaces can achieve multidimensional interplay with the host environment, and foster a microenvironment that is conducive to both vascularization and osteogenesis. This intricate interplay is underpinned by the synergistic effects between bioactive components including the growth factors and signaling molecules, intricately regulating cellular responses. Notably, these modifications can serve as bioactive cues and direct cellular activities towards the direction of enhancing angiogenic and osteogenic potential. The modified surfaces of bone implants, acting as a dynamic interface with surrounding tissues, stimulates endothelial cells to facilitate neovascularization while concurrently promoting the adhesion and differentiation of osteogenic cells. Moreover, the controlled release kinetics of bioactive components from the implant surface can contribute to sustained and localized effects, further optimize the tissue-regenerative milieu. This biofunctionalized approach holds considerable promise for developing smart implants that seamlessly integrate with host tissues and actively achieve angiogenesis and osteogenesis, which ultimately advances the development of bone tissue engineering.
3.3.3. External responsive implants for enhancing osteogenesis and angiogenesis
Responsive implants can respond to the exogenous physicochemical factors such as light, magnetism, ultrasound, temperature, pH, etc. and trigger a series of reactions and/or property transformation in response to reinforce their interactions with cells and biological activities for finally better bone repair and remodelling effects [171,305]. For example, mild exogenous heat stimulation, 2–4 °C higher than the normal body temperature, can remarkably intensify osteogenic capacity including the ALP expression and the mineralization ability of BMSCs [306,307]. However, the first biological window (NIR-I, 650–950 nm in wavelength) inherently has poor tissue penetration depth due to scattering in biological tissues, which restricts the use of some photothermal conversion agents [308,309]. It has recently been demonstrated that the second NIR window (NIR-II, approximately 1000–1700 nm in wavelength and a section of shortwave-infrared) can provide additional advantages, including deeper penetration into biological tissues, less tissue scattering or absorption, and decreased interference by fluorescent proteins time monitoring ability, which provides a much more advanced choice compared to both the visible and NIR-I windows. The Yang group recently engineered the 3D-printed PCL composite scaffolds combined with wesselsite [SrCuSi4O10] nanosheets, a NIR-II (1000–1700 nm in wavelength) photothermal conversion agent, which manifests tremendous vascularized bone regeneration in vivo of rat models [310]. Under the photothermal effect generated by NIR-II, the novel scaffolds not only promote the proliferation and differentiation of BMSCs and HUVECs but also boost thermal ablation of deep-seated osteosarcoma via hyperthermia effects. Simultaneously, the formation of neo-bone with more neovascularization is also elevated with the sustained release of Sr, Cu, and Si ions from the scaffolds.
In addition to the photothermal effect created by the photodynamic, the thermal effect delivered by the magnetic field also has a greater promoting impact on deep tissue repair. For example, Wang et al. successfully engineered the core-shell structured magnetic iron oxide NPs (CoFe2O4@MnFe2O4) coated with Arg-Gly-Asp (an osteoinductive peptide), which encapsulates in agarose to construct an osteoinductive and angiogenic nanoparticle-hydrogel composite (Fig. 16a) [311]. Under the effect of alternating magnetic field, the mild magnetocaloric effect (41–42 °C) induces the heat stress that facilitates the expression of heat shock protein 90 (HSP90), a stabilizer of p-Akt, which efficaciously activates the PI3K/Akt pathway and then facilitates biomineralization. At the same time, HSP90 also upregulates the expression of HIF-1α through synergizing with cobalt ions released from the NPs, which is conducive to promoting neovascularization. In rat calvarial defect model, magnetothermal NPs hydrogel can effectively promote osteogenic and angiogenic in vivo effects (Fig. 16b and c). The multifaceted approaches integrate the external stimuli with advanced biomaterials, and further enhance the potential for tailored interventions in bone regeneration. By exploiting exogenous factors like heat and magnetic fields, researchers strategically modulate cellular responses and signaling pathways, thereby orchestrating a finely tuned cascade of the vascularized osteogenic microenvironment that culminates in enhanced osteogenesis and angiogenesis.
Fig. 16.
Implants-mediated vascularized osteogenic microenvironments. (a) Schematic diagram of the synthesis of core-shell-structured magnetic iron oxide NPs (MIONs)/Agarose (MRA) hydrogels, and its mechanism of osteogenesis, biomineralization, and angiogenesis in the bone defect site under mild magnetic hyperthermia. (b) Relative angiogenic gene expressions of endothelial cells after culturing with Fe3O4-Arg-Gly-Asp (RGD), MnFe2O4-RGD, and MION-RGD for 3 days. (c) 3D reconstruction images by micro-CT scanning showing new blood vessels at the defect site (upper panel) and micro-CT images of new bone formations in the cranial bone defects of the rats in 8 weeks post-surgery (lower panel) [311]. Copyright 2022, Elsevier. (d) Mechanistic insight into the DBM/GP scaffold-induced osteogenesis and angiogenesis. The nanofibrous DBM/GP scaffold with interconnected microchannel networks could promote angiogenesis and osteogenesis by the sequential release of Si, angiogenic DMOG, and osteogenic BFP-1. (e) Digital photos and 3D reconstructed micro-CT images of neovascularization at 4 weeks after subcutaneous implantation of scaffolds (scale bar: 5 mm). (f) Representative 3D-reconstructed micro-CT images in the defect sites after scaffold implantation for 12 weeks including transverse and coronal sections. The colour mapping indicated that the higher degree of red colour correlated with the higher degree of mineralization [312]. Copyright 2022, Wiley-VCH GmbH.
3.3.4. Implants-mediated vascularized osteogenic microenvironments
The bone microenvironment is crucial for osteogenesis and bone defect repair, however, simulation of the bone microenvironment through implant materials is still a challenge. Surprisingly, Ha et al. successfully fabricated a microchannel networks-enriched nanofibrous gelatin-silica scaffold that possessed the bone microenvironment-mimetic microchannel structures and facilitated cell migration and nutrient transportation (Fig. 16d) [312]. With the release of dimethyloxalylglycine (DMOG) and BFP-1, the dual-drug delivery nanofibrous hybrid scaffold (DBM/GP) enhances the vascularization ability of osteoblasts by activating PI3K/Akt/HIF-1α pathway. Simultaneously, the scaffold mimetics the bone microenvironment that boosts vascularized bone regeneration through stimulating tube formation and angiogenesis-related genes/protein expression of endothelial cells and synergistic osteo-related genes expression and mineral deposition of osteoblasts (Fig. 16e and f). For the smart implants, the desirable elastic modulus and density are essentially similar to the heterogeneous microstructures and mechanical properties of natural bone, however, the bone ECM is also important for orchestrating the osteogenic microenvironment [313,314]. For instance, Ma et al. developed the bioinspired GelMA/Ti6Al4V hybrid scaffold that effectively mimics the ECM, which is complementary to its microstructure to ameliorate the microenvironment of bone defect repair [315]. With a 10 % (14.46 ± 1.55 kPa) concentration of soft hydrogel in it, the hybrid scaffold exhibits a favouring milieu for the greatest ability to facilitate BMSCs adhesion and differentiation. The scaffold's dual bionic design also promotes bone and vascular regeneration by upregulating genes associated with osteogenesis and angiogenesis through the modulation of the PI3K/Akt/mTOR pathway. Researchers also design the hybrid scaffold via combining the PCL, fibrin, and HUVECs-derived decellularized ECM, which reconstitutes a bio-instructive microenvironment for vascularized bone regeneration [316]. The decellularized ECM consists of a variety of angiocrine biomolecules that mediate crosstalk between endothelial cells and osteoprogenitors, facilitating the coupling of angiogenesis and osteogenesis. Additionally, it simulates the bio-instructive bone milieu, significantly enhancing the formation of type H vessels and exhibiting superior endogenous osseointegration effects.
Among the HIF family, HIF-1α, a transcription factor that promotes angiogenesis by simulating hypoxic conditions and promoting the transcription of VEGF, plays an important role in maintaining bone remodelling as well as neovascularization. Previous studies have shown the hypoxic microenvironment as the response to target the cellular HIF-1α pathway, which results in the activation of numerous pro-angiogenic genes including VEGF, etc., and then stimulates neovascularization [317,318]. As bone is a relatively hypoxic region, the related host bone tissue will be rapidly depleted of oxygen after implantation [319,320]. Thus, insufficient oxygenation can cause a range of issues and the hypoxic microenvironment between implant and vascularization of host tissue is almost inevitable. Self-oxygenation of tissues can transform hypoxic stresses into anoxic stimulation, which provides a new strategy for exploiting the hypoxic microenvironment to promote advanced tissue regeneration [321]. For example, Yang et al. used 3D printing technology to construct an enzyme-functionalized scaffold constituted with alginate, glucose oxidase, and catalase-assisted CaP nanosheets [322]. In the presence of a hyperglycemia environment, the glucose and oxygen are catalyzed by glucose oxidase into the gluconic acid and H2O2, while the catalase-assisted CaP nanosheets can alleviate inflammation in diabetes mellitus by scavenging the generated H2O2. This ultimately results in a hypoxic microenvironment that can stimulate neovascularization, meanwhile, the degraded Ca2+ and PO43− ions from catalase-assisted CaP nanosheets can facilitate the vascularized bone regeneration.
The implants-mediated vascularized osteogenic microenvironment represents a multi-dimensional system that intricately regulates bone formation around implants. Currently, numerous studies are exploring the intricate mechanisms governing the vascularized osteogenic microenvironment, aiming to decipher the molecular mechanisms that orchestrate bone tissue regeneration between implanted materials and host tissues. Surface modifications of the implant materials play a decisive role in modulating cellular responses and promoting osteogenic and angiogenic activities. The controlled release of bioactive molecules from the implant surface facilitates a favourable microenvironment, guiding cell behaviour and tissue regeneration. Moreover, the recruitment and differentiation of endothelial progenitor cells contribute significantly to the vascularization of the implant site, enhancing nutrient supply and waste removal. In conclusion, the establishment of a vascularized osteogenic microenvironment is paramount for the success of bone implantation. The meticulous study of bioactive implants not only enhances our understanding of fundamental vascularized osteogenic processes but also provides valuable insights for the development of more advanced bone implants with enhanced regenerative capabilities.
4. Comprehensive functions of smart bone implants
The different composite biomaterials are increasingly being employed in bone tissue engineering, aiming to closely mimic the natural osteogenesis microenvironment. Compared with traditional implants, the smart osteogenic biomaterials often endow distinct advantages, including the efficient osteogenic ability combined with antibacterial, angiogenic, or immunomodulatory properties. These attributes play a crucial role in bone tissue repair and remodelling. Despite the success of bifunctional platform-based smart implant materials, numerous challenges also persist in achieving optimal bone healing. Complex clinical issues, such as septic prosthetic joint loosening, cannot be attributed to a single factor alone but rather result from multiple interrelated aspects, including bacterial invasion, inflammatory responses, immune regulation, and interactions between osteoprogenitor cells and various cell types [26]. Notably, comprehensive functional biomaterials that simultaneously integrate basic osteogenic properties with at least two additional functions-such as antimicrobial, angiogenic, immunomodulatory, or neuroregulatory effects-hold great promise for addressing clinical complications in bone healing. These include delayed union, nonunion, large bone defects, poor vascularization, and periprosthetic infection, while also mitigating the growing threat of antibacterial drug resistance [27,139,[323], [324], [325]]. Consequently, the multifunctional osteogenic properties of smart implants may eventually achieve the effect of killing several birds with one stone, significantly improving bone-implant osseointegration and accelerating bone tissue repair and regeneration (Table 3).
Table 3.
The comprehensive functions of bone implants.
| Biomaterial type | Smart composition | Advantage |
Model | Ref. | |
|---|---|---|---|---|---|
| Common | Special | ||||
| Bactericidal activity, immunomodulation, and osteogenesis | |||||
| nano-painting Ti | CaO2 nanoparticles, Cu2+, •OH | 1) destroy bacterial membranes, 2) modulate polarization of M2-phenotype macrophages, 3) enhance osteogenesis and osseointegration |
Acidic microenvironment response, Fenton-like reaction, ameliorate hypoxic microenvironment | MRSA, BMSCs, BMDMs | [332] |
| surface modified Ti | OGP-loaded ZIF-67, Co2+, | acidic environment-sensitive property, formation of an alkaline microenvironment, | E. coli, S. aureus, MRSA, RAW264.7 cells, rat BMSCs | [333] | |
| surface modified Ti | plasmid-laden peptide nanoparticle, short hairpin RNA for caspase-11 | generation of super CAR-macrophages | MRSA, mouse BMDMs | [337] | |
| Bactericidal activity, angiogenesis, and osteogenesis | |||||
| nanoglass paste | Ca2+, Cu2+, silicate ions | 1) destroy bacterial membranes, 2) enhance osteogenic differentiation of BMSCs, 3) stimulates HUVECs proliferation, upregulate HIF-1α and VEGF |
self-hardened, promote bacterial intracellular ROS level | E. coli, S. aureus, rat BMSCs, HUVECs | [342] |
| surface modified Ti | CuS@BSA, rGO, PTT treatment | 1O2 generation, recruitment BMSCs, | E. coli, S. aureus, mouse BMSCs, HUVECs | [344] | |
| collagen–nanohydroxyapatite scaffolds | antagomiR-138-activated copper-doped bioactive glass particles, | induce osteogenic–angiogenic coupling | E. coli, S. aureus, human BMSCs, shell-less chicken embryo model | [345] | |
| surface modified Ti | HHC36 antimicrobial peptide, QK angiogenic peptide | mimic VEGF protein | E. coli, S. aureus, MRSA, HUVECs, human BMSCs | [348] | |
| Angiogenesis, immunomodulation, and osteogenesis | |||||
| bilayer bone regeneration membrane | hierarchical mineralized collagen, Zn2+ | 1) promote macrophage M2 polarization, 2) stimulate the osteogenic differentiation of BMSCs, 3) enhanced the proliferation and differentiation of HUVECs | bone-like hierarchical structure, controls the release of Zn2+ | human BMSCs | [355] |
| 3D-printed scaffolds | GHK-Cu tripeptide complex, | paracrine effects of VEGF and BMP2 | rat BMSCs, RAW264.7 cells, HUVECs | [356] | |
| HA/polylactic acid membrane | HA nanowires, stromal cell-derived factor-1 | sustained release, recruitment of endogenous cells | human periodontal ligament stem cells, mouse BMDMs | [357] | |
| 3D-printed PCL scaffolds | EGCG, Si ions, surface topography | ROS-scavenging | rat BMSCs, RAW264.7 cells, HUVECs | [358] | |
| biomimetically hierarchical scaffold | MnCO, DFO@PCL NPs, PLA/HA | persistently produced CO and Mn2+, suppress osteoclast differentiation | rat BMSCs, RAW264.7 cells, HUVECs | [361] | |
| Bactericidal activity, angiogenesis, immunomodulation, and osteogenesis | |||||
| surface modified PEEK | Cu2+, Cu-enriched microenvironment | 1) destroy bacterial membrane, 2) early pro-inflammatory response, 3) late anti-inflammatory response, 4) enhance angiogenic and osteogenic capabilities | elevated ROS level | MRSA, RAW264.7 cells, rat BMSCs, HUVECs | [366] |
| 3D-printed composite hydrogel scaffold | Cu2+, Ca2+ and Si2+ ions, | spatiotemporally coordinated immunomodulatory | E. coli, S. aureus, HUVECs, RAW264.7 cells, rat BMSCs | [370] | |
| Neuroregulation, angiogenesis, and osteogenesis | |||||
| Composite hydrogel scaffold | BP@Mg, β-TCP | 1) increasing nerve-related protein expression, 2) enhance the migration and tube formation of endothelial cells, 3) promote osteogenic differentiation of BMSCs | sustained release | rat BMSCs, HUVECs, Mouse neuroectodermal stem cells NE-4C, PC12 cells | [385] |
| 3D bioprinted composite hydrogel scaffold | CGRP, PRN, micro-nano hierarchical structure | simulate secretion of neuropeptides | HUVECs, rat BMSCs | [386] | |
| extracellular matrix scaffold | NGF, paracrine CGRP | induce neurotrophic microenvironment, regulate NGF-TrkA signaling pathway, self-adaptive modulation of skeletal homeostasis | schwann cells (SCs), rat aortic endothelial cells, rat BMSCs, human BMSCs | [371] | |
| 3D bioprinted scaffold | SC-exos, BMSCs | mimic SCs-mediated nerve–bone crosstalk, upregulate TGF-β signaling pathway | rat BMSCs, endothelial progenitor cells, SCs | [387] | |
4.1. Multifunction of bactericidal activity, immunomodulation, and osteogenesis
The risk of implant-associated infections is increasing, and the designing of biomaterials with osteogenic and antibacterial properties for bone tissue regeneration showed considerable effects. However, the emergence of antibiotic resistance, the “race for the surface” model, the presence of intracellular bacteria, and the dysregulation of immune cell subset protection all highlight the critical role of host immunomodulation in anti-infection responses [143,[326], [327], [328]]. With a focus on host immune function, the immunomodulatory antimicrobial biomaterials can potentiate the host defence mechanisms via immune-protective properties, which functionalizes the bone implants with excellent anti-infective and osteogenesis properties [329,330].
The bacteria-mediated microenvironments around the implants have been proven to adversely affect immune regulation and osseointegration. At the same time, the bacterial biofilm consumes much of the environmental nutrients, leading to a further impaired immune response [331]. It is necessary to engineer the multifunctional implants to address this tricky tissue. For example, Zhang et al. designed a bioinspired nano-painting on orthopaedic implants for simultaneously promoting osseointegration and preventing infection, which used the polyphenol-functionalized CaO2 nanoparticles to decorate Ti implants via both the interfacial molecular interactions of mussel-bioinspired adhesion and Cu2+ coordination-based interparticle lock [332]. When faced with an acidic condition produced by bacteria, the smart coating could be rapidly disassembled and produced •OH by the released Cu2+ mediated Fenton-like reaction to eradicate bacteria and biofilm. With the release of O2 from CaO2, the hypoxic microenvironment is improved, which can induce pro-inflammatory polarization of macrophages through collaborating with Cu2+ ions. Ultimately, with the synergistic effect of immunomodulation and the released Cu2+ and Ca2+, the novel implant significantly prevented periprosthetic infection and reinforced osteogenesis and bone-implant integration in an arthroplasty rat model. Compared to bacteria-induced acidic microenvironments, the appropriate alkaline milieu induced by implants can significantly prohibiting bacterial survival and enhance the proliferation and ALP activity of osteoblasts [7,168]. Therefore, this special microenvironmental characteristic is employed by some researchers to construct smart multiple biomaterials with both regenerative and anti-infective properties. For example, the osteogenic growth peptide-loaded zeolitic imidazolate frameworks-67 decorated titanium dioxide nanotubes coating is successfully prepared, which could rapidly dissolve with an acidic environment-sensitive property [333]. The special biomaterial may destroy bacterial membranes early in the implantation process due to the release of Co2+ and the formation of an alkaline microenvironment. Simultaneously, the inflammatory response was suppressed, modulating the polarization of M2-phenotype macrophages to stimulate the cellular differentiation of BMSCs, thereby enhancing the osteointegration of the bone implant through synergizing with osteoimmunomodulatory and antibacterial effects at the next stage.
Macrophages, the key component of the innate immune system, play a critical role in responding to periprosthetic joint infection by initiating their antimicrobial activity upon internalizing the pathogen. Nowadays, the chimeric antigen receptors (CARs) represent a pivotal technology in the emerging field of immunocellular therapy, which involves the modification and reactivation of immune cells to exert targeted killing of pathogens [334]. The CARs consist of an extracellular antigen-targeting domain, typically a single-chain variable fragment, and an intracellular signaling domain, usually derived from the T-cell receptor that provides an excellent immunotherapeutic strategy for activating the innate immunity to treat bacterial infections [335,336]. Li et al. engineered a plasmid-laden peptide nanoparticles (pPNP)-coated Ti implant that introduced MRSA targeted CAR genes and short hairpin RNA for caspase-11 into macrophage nuclei, resulting in the generation of super CAR-macrophages for locoregionally antimicrobial and periprosthetic osseointegration (Fig. 17a–d) [337]. With the increased presence of CAR-modified macrophages around the bioactive implant, the implant indeed achieves robust phagocytosis and eradication of MRSA. Meanwhile, the in vivo degradability of the pPNP coating precisely matches the process of bone regeneration in a periprosthetic joint infection mouse model, achieving satisfactory osteogenesis and expediting peri-implant osseointegration. In summary, this pioneering study not only elucidates the intricate interplay between engineered CAR-macrophages and the targeted eradication of bacterial threats, but also underscores the strategic integration of biomaterials in promoting successful osseointegration. These findings hold promising implications for the advancement of immunocellular therapy, specifically in the context of periprosthetic joint infections. As the field progresses, the utilization of chimeric antigen receptors and innovative biomaterials continues to pave the way for enhanced strategies in combatting infections and promoting the long-term success of orthopaedic implants.
Fig. 17.
Multifunctional platforms for synergistic antibacterial and osteogenic effects via immunomodulatory and angiogenic strategies at the bone-implant interface. (a) Schematic illustration of the locoregional generation of S.aureus-specific super CAR-macrophages (MΦs) at the bone-implant interface for preventing periprosthetic joint infection. (b) Representative images of the phagocytosis of MRSA by macrophages treated with each formulation. (c) Representative images of the intracellular survival of MRSA in bone marrow-derived MΦs (multiplicity of infection = 5:1) in the indicated treatment groups. Scale bars is 2 cm. (d) Representative Masson staining (blue, bone tissue) for the neonatal bone surrounding the implants. Scale bars are 200 (upper panel) and 50 μm (lower panel), respectively [337]. Copyright 2023, American Association for the Advancement of Science. (e) Schematic illustration of synergistic photocatalytic antibacterial and osseointegration via coupling CuS@BSA NPs and reduce graphene oxide (rGO) without biologics. CuS@BSA and rGO demonstrate excellent antibacterial property via hyperthermia and 1O2 generation under NIR radiation. Cu ions derived from CuS@BSA promotes angiogenesis and cooperates with rGO to promote vascularized bone regeneration [344]. Copyright 2021, Elsevier.
4.2. Multifunction of bactericidal activity, angiogenesis, and osteogenesis
Implants-associated infection causes serious problems in the healing process of bone tissue. Fortunately, antimicrobial Ti implants have been demonstrated that can achieve regenerative therapy in the face of biomaterial-associated infection [338]. However, due to the uncertain cytotoxicity of antimicrobial implants, it would impair the biocompatibility, angiogenic activity, and osteogenic activity of the implants [[339], [340], [341]]. The antimicrobial resistance risks and large bone defects following infected tissue removal are increasing after long-term antibiotic treatment. The optimal solutions are always explored by researchers to address bacterial infections without relying on prolonged antibiotic use. Consequently, there is a pressing need to engineer implantable biomaterials to relay osteogenesis and angiogenesis regenerative abilities and contemporaneously effectively fight bacteria.
The multiple therapeutic actions of the smart implantable materials will provide a better solution for clinical work to reduce the dose of antibiotics and related side effects. For example, Seo et al. developed a practical Cu-nanoglass paste consisting of ∼200 nm silicate-glass particles with Ca2+ and Cu2+, which would be self-hardened in contact with an aqueous medium [342]. The shapeable nanomaterials provide an excellent therapeutic strategy for the possession of bacteria-infected and defective bone tissues, especially irregular bone defects. As the innate characteristics of the bioactive nanoglass paste, the silicate and calcium ions were released to promote osteogenic differentiation of BMSCs and osteoblast biomineralization while inducing vasculogenesis via upregulating HIF-1α and VEGF. Simultaneously, with the release of Cu ions and internalized bacteria, the intracellular ROS level is drastically increased while the synthesis and transport of amino acid are inhibited, which destroys membrane integrity and leads to the effective killing of bacteria. Interestingly, the Cu ion is also been considered to play an additive or synergistic role with silicate ions in osteogenesis and angiogenesis in vitro [342,343]. Similarly, other studies also create self-assembly coatings with BSA-modified copper sulfide NPs (CuS@BSA) and reduce graphene oxide (rGO) on implant surface, which not only demonstrate excellent coupled photothermal sterilization with vascularized osseointegration capability but also avoid tissue damage caused by hyperthermia during photothermal therapy treatment (Fig. 17e) [344]. To begin, the release of Cu2+ from CuS@BSA stimulates angiopoiesis by intensifying the proliferation and differentiation of HUVECs and promoting microtubule formation. Meanwhile, the CuS/rGO decorated surface couples with the enhanced photothermal effects and generation of 1O2, and substantially enhances the antibacterial efficacy of implants by 40 times. Furthermore, Sadowska group developed the multifunctional collagen-nanoHA scaffolds incorporated with antagomiR-138-activated Cu-doped bioglass particles for antimicrobial treatment and vascularized osteogenesis [345]. This multifunctional scaffold leverages the intrinsic bactericidal properties of Cu ions, reducing the adherence of gram-positive bacteria by over 80 %, thus demonstrating substantial antimicrobial efficacy. Simultaneously, it stimulates bone healing and remodelling through osteogenic-angiogenic coupling induced by antagomiR-138 NPs, achieving the infectious bone repair strategy with antibiotic-free biomaterial and gene therapy.
In addition, immobilization techniques for antimicrobial peptides, incorporating the peptide agents into implant biomaterials, have also been demonstrated to be effectively antibacterial treatment methods [346,347]. Notably, Chen et al. innovatively designed the fusion peptide that consists of the HHC36 antimicrobial sequence (seq: Lys-Arg-Trp-Trp-Lys-Trp-Trp-Arg-Arg) and QK angiogenic sequence (seq: Ile-Gly-Lys-Tyr-Lys-Leu-Gln-Tyr-Leu-Glu-Gln-Trp-Thr-Leu-Lys) [348]. The fusion peptide is used to modify the Ti implant that achieves an antibacterial efficiency of 96.8 % against the common clinical bacteria, which is ascribed to the larger bacterial accessible surface area of the HHC36 sequence. Simultaneously, the VEGF-mimetic peptide QK functions similarly to the VEGF protein in inducing osteogenesis and coupling angiogenesis to osteogenesis [349]. Therefore, the innovative implant significantly inhibits bacterial infection and promotes vascularization and osseointegration without any external stimuli. These multifunctional smart implants innovatively provide promising strategies for clinical problems such as implant infections.
4.3. Multifunction of angiogenesis, immunomodulation, and osteogenesis
The inflammatory response is inevitable with the implantation of biomaterials, which can impair the osteointegration of implants and follow osseous regeneration [214]. How to achieve the appropriate immune microenvironments of bone implants is crucial for bone tissue regeneration. At the same time, the predominant M2 phenotype macrophage and favourable osteoimmunomodulatory properties not only exhibit excellent anti-inflammatory activity but also further promote synergistically angiogenesis and osteogenesis via secreted factors, such as VEGF, PDGF-BB, BMP-2, BMP-4, TGF-β, etc [230,350]. Therefore, promoting the creation of a pro-regenerative immune milieu surrounding the implant biomaterial site is extremely beneficial to accelerate vasculogenesis and bone repair.
Currently, many researchers have turned their attention to the benefit of the immune cells in the process of vascularized bone regeneration. It is a wise idea to start with the biomaterial itself to create a favourable osteoimmunomodulatory microenvironment that will facilitate osteo/angio-genesis. To meet the rapid, satisfactory, and multilevel requirements of osseointegration, Bai et al. developed a biomimetic coating with micro/nanoscale TiO2 fibres-like network on the surface of Ti implants, which is comparable ECM architecture to the host bone tissue at healing stage [351]. The biomimetic micro-nano structure creates a favourable osteoimmune milieu, which enhances the M2 polarization of macrophages and lessens inflammation response through down-regulation of the inflammatory cytokines and activation of autophagy. Meanwhile, the special micro-nano structure also stimulates the osteocytes to produce more pseudopodia and sustains the HUVECs vitality based on the appropriate roughness and hydrophilicity [351,352]. Continuing from the material design aspect, the bioactive ions (e.g., Sr2+, Ca2+, Zn2+, Cu2+, Mg2+, etc.) incorporated with implants or other materials with the appropriate concentration ranges are also desirable strategies for immune and vascular-mediated bone regeneration [274,277,353,354]. Recently, Yan et al. developed a bilayer self-induced endogenous bone regeneration membrane (ss-HMC/Zn) by combining biodegradable Zn with hierarchical mineralized collagen (HMC) to address maxillofacial bone defects [355]. This novel membrane forms a bone-like hierarchical structure and effectively controls the release of Zn2+, which further induces M2 macrophage polarization for osteoimmunomodulation. Simultaneously, it promotes the recruitment of endothelial progenitor cells (EPCs) and BMSCs, facilitating vascularized bone regeneration in maxillofacial defects without the need for osteogenic supplements. Zhou et al. developed 3D-printed silk-based scaffolds with copper peptide, a glycyl-l-histidyl-l-lysine-Cu tripeptide complex (GHK-Cu), which rendered an effective strategy utilizing bioactive Cu2+ for the enhancement of vascularized bone tissue repair and remodelling [356]. Through the sustained release of GHK-Cu, the Cu-related capacities of anti-inflammatory response and promotion of macrophage M2 polarization are markedly augmented during the initial phases of bone tissue regeneration. Concurrently, the copper peptide not only stimulates the osteogenic differentiation of BMSCs but also facilitates the vascularized bone regeneration through modulating the paracrine effects of VEGF and BMP2. Likewise, researchers have also utilized the multifunctional agent of stromal cell-derived factor-1 (SDF-1) to embellish the HA nanowires, subsequently integrating them into the multifunctional HA/PLA membrane. This innovative approach expedites the recruitment of endogenous cells and facilitates the polarization of macrophages toward the M2 phenotype in the early stages of bone repair [357]. The sustained release of SDF-1 from the HA/PLA membrane extends its impact into the later stage of bone repair, which simultaneously facilitates vessel formation and enhances mature bone regeneration. This dynamic and sustained of regulatory strategies underscore the significant potential in creating favourable and enduring osteogenic conditions for the regeneration of bone tissues.
Given the advantages of comprehensive multifunctional biomaterials, Xiao et al. have pioneered the fabrication of multifunctional bionic coatings on 3D-printed PCL scaffolds via the LbL self-assembly technique, meanwhile, the epigallocatechin gallate and polyethyleneimine aggregates facilitate the SiO2 precipitation as bridges of silicon coupling (Fig. 18a) [358]. The SiO2-coated scaffolds exhibit prominent intracellular ROS-scavenging and anti-inflammatory activities that is ascribed to the reaction between the polyphenol epigallocatechin gallate and free radical species, which then modulates the immune microenvironment for macrophages inclined to the M2 phenotype. Simultaneously, the release of Si ions and the synergistic effects of surface topography significantly enhance the proliferation and differentiation of angiogenic and osteogenic cells, along with the neonatal bone generation in the defect area (Fig. 18b–e). This acceleration of vasculogenesis and osteogenesis was achieved by activating various intracellular signaling pathways including ECM interaction, PI3K, MAPK, HIF, and TGF signaling pathways. Importantly, the induced immune microenvironment plays a pivotal role in supporting angiogenesis and osteogenesis. The intricate orchestration of the immune microenvironment emerges as a pivotal factor in driving vascularized bone regeneration. Within this dynamic setting, the immunomodulatory activities induced by the multifunctional biomaterials contribute significantly to fostering an environment that is conducive to the formation of functional vasculature and robust bone tissue.
Fig. 18.
Multifunctional coating strategy for promotion of angiogenesis, immunomodulation, and osteogenesis. (a) Schematic illustration of the preparation process and osteogenesis-related multifunction of SiO2 coating on 3D-printed PCL scaffolds induced by epigallocatechin gallate and polyethyleneimine (EGCG/PEI) layers. The EGCG incorporated in coatings exhibits anti-oxidant and immune regulation activity and the release of Si ions facilitates the osteogenic and angiogenic differentiation of BMSCs and HUVECs, respectively. (b) SEM images of RAW264.7 cells after incubation with scaffolds for 1 and 3 days. (c) Gene expression of iNOS and CD206 in RAW264.7 cells incubated with the conditional medium for 1 day. ∗p < 0.05 and ∗∗p < 0.01. (d) The tube formation of HUVECs after incubation on different surfaces for 6–24 h. (e) The 3D Micro-CT reconstruction images of the femoral condyle and new bone tissue in the defect area at 4 and 12 weeks after implantation [358]. Copyright 2022, Wiley-VCH GmbH.
Within the intricate landscape of cellular responses, the carbon monoxide (CO) can serve as a distinctive endogenous signaling molecule, which has been shown to exhibit a propensity for polarization towards the anti-inflammatory phenotype of macrophages within the immune microenvironment. This unique property enables CO to effectively suppress inflammation at the source, emphasizing its potential as a modulator of the immune microenvironment in orchestrating favourable conditions for tissue repair and regeneration. [359,360]. Recently, Zhang et al. innovatively engineered an osteoimmunity-regulating and biomimetic hierarchical scaffold for ameliorating large-scale bone defect repair, which consists of the deferoxamine@PCL NPs, manganese carbonyl (MnCO) nanosheets, GelMa hydrogel, and PLA/HA matrix [361]. Interestingly, the CO and Mn2+ can be persistently produced locoregionally through a Fenton-like reaction between MnCO and endogenous H2O2, which can polarize macrophages towards the M2 phenotype and then alleviate the inflammatory response. Simultaneously, Mn2+ and deferoxamine@PCL NPs significantly promote neovascularization via activating the HIF-1α pathway and synergizing with macrophage-derived VEGF. Moreover, the deferoxamine can suppress osteoclast differentiation to some extent and cooperate with the osteoinductive activity of HA, which finally facilitates new bone formation. The explore of these innovative scaffolds not only confirms the synergistic effects of angiogenesis, immunomodulation, and osteogenesis, but also introduces a novel dimension for bone tissue engineering by incorporating the regulation of osteogenic immune microenvironment.
4.4. Multifunction of bactericidal activity, angiogenesis, immunomodulation, and osteogenesis
Undoubtedly, the indispensability of antibacterial, angiogenic, immunomodulatory, and osteogenic activities play pivotal roles in the intricate process of bone tissue repair and regeneration, as highlighted previously. The healing of bone tissues is a protracted, continual, and dynamic process, and the specific functions demanded by the body in various repair stages may indeed exhibit subtle variations [[362], [363], [364], [365]]. During the intricate procession of bone tissue regeneration, each stage mandates a nuanced orchestration of biological responses to meet evolving requirements. In the initial phases, the indispensable role of antibacterial property is highlighted for mitigating infection risk and safeguarding the healing environment. The angiogenesis simultaneously shows paramount importance, fostering the establishment of a robust vascular network that is crucial for nutrient supply and waste removal of bone tissues. As the repair process advances, the focus of osseointegration continually sharpens on immunomodulatory facets. A balanced immune response becomes imperative to regulate inflammation, facilitate tissue remodelling, and pave the way for subsequent healing stages. Subsequently, particularly noteworthy is that the intricate immunomodulation can then steer macrophages towards an anti-inflammatory M2 phenotype, aligning with the dynamic needs of regenerating tissue. Bone regeneration behaviour concurrently takes the centre stage in the later phases of bone repair. The orchestration of various osteogenic properties becomes critical for the formation of robust bone tissue, ensuring structural integrity and functional recovery. The collaborative interplay of antibacterial, angiogenic, immunomodulatory, and osteogenic functionalities can better tailor to the variable demands of each healing stage for successful bone tissue repair and regeneration. Obtainment of the smart implants can comprehensively address these miscellaneous conditions and hold great promise in advancing the field of regenerative medicine for bone-related applications.
As a multifunctional bone implant, the excellent antibacterial and vascularized osteogenic abilities are crucial for treating clinically prevalent prosthesis infection and loosening. For example, the multifunction PEEK implant is successfully fabricated, which is coated by the PDA and Cu by mussel-inspired metal-catecholamine assembling strategy [366]. The presence of Cu-containing groups on the coating facilitates the formation of a Cu-enriched microenvironment around the implantation site. This microenvironment not only demonstrates direct antibacterial activity through membrane damage and elevating the ROS levels, but also induces macrophage polarization to a pro-inflammatory M1 phenotype in the early stage. Surprisingly, recent researches has further unveiled that an early pro-inflammatory response modulated by the M1 macrophages, can create a favourable inflammatory microenvironment for osteogenesis [247,367]. The Cu ions play a crucial role in stimulating blood vessel formation, maturing bone collagen, and promoting the osteogenic differentiation of BMSCs. Simultaneously, the favourable immunomodulatory effect collaborates with the direct function of Cu2+, effectively promoting vasculogenesis and further enhancing osteogenic and antibacterial abilities. The innovative design strategies of immunoregulatory implants seamlessly combines osteogenesis, angiogenesis, and antibacterial capabilities, which can achieve bone tissue defect healing that is closer to natural bone functions.
The effective reconstruction of bone tissues, particularly the healing of infected bone defects, is of paramount importance as it not only restores structural integrity but also mitigates the risk of persistent infections, which finally fostering the advanced physiological functions and long-term patient well-being [368,369]. The integration of pathogen clearance, immune modulation, angiogenesis, and osteogenic characteristics within the implant inevitably emerges as crucial for addressing the complex issue of healing infected bone defects. Jian et al. recently designed the dual-photo crosslinked hybrid hydrogel composed of Cu-doped bioglass/methacryloyl-modified gelatin NPs/methacrylated silk fibroin for repairing the infected bone defects (Fig. 19) [370]. In the absence of antibiotics or exogenous recombinant proteins, the functional hydrogel shows outstanding biophysical adhesion, adaptation to irregular defect shapes, and in situ physical reinforcement. The induction of bacterial oxidative stress and membrane depolarization are achieved with the continuous release of Cu2+, can effectively inhibit and kill the S. aureus and E. coli. Importantly, through the sequential release of biological biomimetic signals including Ca2+ and Si2+ from the Cu-doped bioglass, the hydrogel spatiotemporally coordinates the immunomodulatory, vasculogenesis, and osteogenesis via upregulating the corresponding genes. In a rat model of infected bone defects, this novel hydrogel induces biological cascade reaction and effectively remove pathogens in the early stage of implantation. The hybrid hydrogel also promotes the polarization of macrophages to the M2 phenotype and ameliorates the osteogenic microenvironment. The seamless integration of antimicrobial, angiogenic, immunomodulatory, and osteogenic functionalities within this smart implant underscores its immense potential for providing a comprehensive and effective treatment strategy in addressing infected bone defects. This innovative approach not only addresses the immediate challenge of infection control but also sets the stage for groundbreaking advancements in bone regenerative medicine.
Fig. 19.
Schematic diagram showing the design principle of the dual-photo crosslinked hybrid hydrogel composed of Cu-doped bioglass (CuBGs)/methacryloyl-modified gelatin NPs (MA-GNPs)/methacrylated silk fibroin (SilMA) and the mechanism of infected bone defect regeneration induced by this hybrid hydrogel through spatiotemporally regulating the healing process [370]. Copyright 2023, Wiley-VCH GmbH.
4.5. Emerging neurogenic bone repair
Recent evidences gradually substantiate that the regulatory impact of the nervous system on both skeletal regeneration and metabolism [12,24,371,372]. The neural regulation of bone regeneration involves a sophisticated interplay between the nervous system and bone tissue, contributing significantly to the intricate process of bone healing and renewal. Nerve fibres play a crucial role in orchestrating various stages of bone regeneration, from initial injury response to the formation of new bone tissue. Upon bone tissue injury, peripheral nerves are activated and initiate a cascade of events that contribute to both bone development and repair by releasing a myriad of signals, including neurotransmitters, neuropeptides, axon guidance factors, and neurotrophins [373,374]. Recently, sympathetic nerves are observed to accelerate bone formation in the early stage of the fracture healing process by modulating the anti-inflammatory environment within the bone marrow [375,376].
Neurogenic regulation also influences angiogenesis by facilitating neurovascular network communication and reconstruction through nerve-derived factors, which are essential for bone repair and regeneration [377,378]. Furthermore, sympathetic nerve activity further impacts bone metabolism by the released neurotransmitters interacting with bone cells, influencing the balance between bone formation and resorption and, consequently, the overall bone remodelling process [379,380]. Stem cells within the bone microenvironment also respond to neural signals, such as neuronal calcitonin gene-related polypeptide-α (CGRP), neuropeptide substance P (NSP), and neuropeptide Y (NPY), influencing their differentiation and participation in bone tissue repair [[381], [382], [383], [384]]. The crosstalk between nerves and stem cells adds additional complexity to the regulation of bone regeneration, which highlights the integral role of the nervous system in shaping the regenerative outcome.
Neural regeneration-angiogenic crosstalk is integral to bone repair, yet the reconstruction of the nerve-vascular network is often overlooked in biomaterial design. Xu et al. constructed a novel Mg2+-modified BP nanosheets were incorporated into the GelMA hydrogel to form an innovative bilayer hydrogel [385]. This periosteum-simulating structure aims to enhance bone repair by fostering angiogenesis and neurogenesis. The upper hydrogel layer, mimicking a periosteal structure, significantly stimulates angiogenesis by expediting endothelial cell migration and concurrently increasing nerve-related protein expression in neural stem cells. Furthermore, the lower hydrogel layer markedly enhances the activity and osteogenic differentiation of BMSCs. The novel bilayer hydrogel scaffolds overall endow a remarkable capacity to enhance early vascularization and neurogenesis, ultimately facilitating bone regeneration and remodelling. This comprehensive approach, which addresses both neural and vascular aspects, holds great promise for advancing bone defect repair and makes a significant contribution to remodelling the neuromodulatory microenvironment to optimize bone healing outcomes. In addition, Guo et al. innovatively fabricated a 3D bioprinted composite hydrogel scaffold loaded with BMSCs and mesoporous SiO2 NPs encapsulated by CGRP and the β-adrenergic receptor blocker propranolol (PRN) [386]. The secretion of neuropeptide secretion by sensory nerves is initiated with the release of CGRP from the scaffold. Simultaneously, the release of PRN inhibits the binding process of catecholamine to the β-adrenergic receptor. This dual action, in conjunction with CGRP and Si ions, synergistically promotes the osteogenic differentiation of BMSCs. Contemporaneously, the pre-designed micro/nanoscale hierarchical structure of scaffold effectively promotes the migration and tube formation of HUVECs, contributing to the advantageous development of a new capillary network. The orchestrated effects also have been anticipated to significantly enhance the repair of a critical-sized cranial defect in a rat model.
Creating a neurotrophic microenvironment conducive to osteogenesis requires orchestrating a milieu with abundant neurotrophic factors that facilitate the growth and osteogenic differentiation of BMSCs. This specialized milieu plays a pivotal role in fostering the intricate interplay between the nervous system and bone tissue, thereby facilitating effective bone formation and regeneration. Zhang et al. devised a non-covalently incorporating nerve growth factor (NGF) into ECM-constructed 3D scaffolds to ameliorate neurotrophic microenvironment for achieving engineered sensory nerves (eSN)-mediated bone regeneration (Fig. 20a) [371]. Firstly, it is identified that new bone formation is highly correlates with NGF-mediated sensory nerve innervation in calvarial defect. The sustained locoregional release of NGF effectively facilitates the differentiation of eSN and CGRP secretion, which, in turn, leads to the osteogenic differentiation of BMSCs via the NGF-TrkA signaling pathway (Fig. 20b–d). Concurrently, the paracrine CGRP secreted by eSN demonstrates the ability to promote endothelial cell mobility, further contributing to vascularized bone regeneration. Notably, when paired with BMP-2 administration, the eSN endow self-adaptive suppression of excessive bone formation and promotion of bone remodelling by activating osteoclasts via a CGRP-dependent mechanism (Fig. 20e and f). Similarly, Wang's group ingeniously constructed a GelMA/SilMA bioprinted scaffold with SCs-derived exosomes and BMSCs, which effectively simulates nerve-bone crosstalk and ameliorates the neurovascularized bone regeneration microenvironment [387]. Significantly, SCs-derived exosomes not only facilitate osteogenesis by modulating the TGF-β signaling pathway via let-7c-5p but also promote the tube formation capabilities of endothelial progenitor cells for new blood vessel sprouting. The biomimetic biological scaffold also endows effective enhancement of neurovascularized bone regeneration in a rat cranial defect model, highlighting the regulatory influence of the bone neurovascular network strategy for promising bone tissue repair. In summary, the neural regulation of bone regeneration (also nerve-bone crosstalk) encompasses a multifaceted interaction between the nervous system, bone tissue, angiogenesis, and stem cells. The budding explores of smart bone implants assisted in the establishment of a neurotrophic microenvironment for osteogenesis emerges as a novel perspective in advancing the field of bone tissue engineering, which also can offer promising avenue for enhancing bone regenerative strategies and improving clinical outcomes.
Fig. 20.
The innovative neurogenic bone repair strategies. (a) Schematic illustration of the engineered acellular scaffold with sustained-release system of NGF (NGF@S) to promote sensory nerve reinnervation at the site of bone tissue injury through NGF-TrkA signaling pathway and reinnervate sensory nerve secretes CGRP to promote osteogenic differentiation of BMSCs and vascular regeneration in tissues. The sensory nerve also regulates osteoblasts and osteoclasts to participate in bone remodelling and guides self-adaptive bone healing. (b) Representative tile scans of H&E-stained images of the cranial defect. Scale bars are 400 μm (upper panel) and 100 μm (lower panel), respectively. (c) Confocal imaging of immunohistochemical staining of NGF expression on day 3 after cranial injury. n = 3 animals per group. (d) Schematic of hypothesis that NGF secreted by macrophage to promote sensory nerve re-innervation, which in turn secrete CGRP to regulate bone regeneration. (e) Representative images of trap staining of cranial bone in different groups (BMP2@S and NGF@S/BMP2). (f) Representative fluorescence images showing pan-neuronal marker TUBB3+ nerve fibres (green) and sensory nerve marker CGRP+ nerve fibres (red) in the cranial bone after scaffold implantation for 30 days [371]. Copyright 2023, Wiley-VCH GmbH.
5. Conclusion and perspectives
Bone tissue injuries are increasing yearly following the “double-edged sword” of the rapid development of modern social technology. Hence, the demand for high-performance implants and clinical therapy techniques are becoming more urgent in the field of bone tissue engineering. This review systematically summarizes the latest strategies and research perspectives of smart implants for bone tissue repair and regeneration by referring to hundreds of relevant publications in the past decade. The diverse patient-oriented osteogenic microenvironments with weak bone self-healing activity, including the osteoporosis, menopause, diabetes mellitus, rheumatoid arthritis, chronic kidney disease, and thyroid dysfunction, are specially faced by clinical bone repair surgeries, which are also the application barriers that need to be focused on breaking through. The most recent advances in regulation mechanism of osteogenesis-related signaling pathways (e.g., BMPs/Smad, MAPK, and AMPK pathways), balance between osteoblastogenesis and osteoclastogenesis, and autophagic flux by smart implants are further concluded and discussed in detail. Notably, the research strategies and synergetic mechanism of multifunctional platform close to that of natural bone, including osteogenesis, antibacterial/bactericidal capacity, immunomodulatory activity, angiogenesis, and even neural regeneration in bone, are systematically summarized and discussed for developing more intelligent implants. This review proposes a latest perspective on smart bone implants from material science to biomedical mechanisms, aiming to provide innovatively technical and theoretical support for their design and clinical applications in bone defect repair.
Even though many successful smart bone implants-driven strategies for bone tissue repair and regeneration have been reported, there are still significant development and challenges that need to be future focused on thinking and overcoming as follows.
-
(1)
The patient-oriented osteogenic microenvironments, especially the pathological conditions that are unfavourable for bone regeneration such as osteoporosis, menopause, diabetes mellitus, and rheumatoid arthritis, are crucial for a bone implant to finally achieve its osteogenesis-related functions even though it has been proven to have robust and intelligent bioactive structure and property. Hence, understanding and ameliorating the changes in physiological functions and their synergistic effects on new bone formation of populations with diverse kinds of diseases, such as burgeoning obesity, is also the indispensable strategy for guiding the design of smart bone implants and translating them into clinical applications.
-
(2)
As the fundamental function in the regenerative healing of bone tissue defect, the osteogenic capacities of implants including the role of their physicochemical characteristics, interface reactions between implants and tissues, and the release of components (e.g., ions and NPs), have be continuously explored by numerous valuable researches for comprehending its mechanism such as the regulation of key signaling pathways, dynamic balance between osteoblastogenesis and osteoclastogenesis, and autophagic flux in osteogenesis. However, it is of vital importance and more challenging to explore the special process and mechanism of promoting bone regeneration in the pathological osteogenic microenvironments of above-mentioned special patients in clinical practices.
-
(3)
Even though many successful manufacturing strategies and functional mechanisms of smart bone implants have been reported, significant challenges remain, particularly in understanding the underlying osteogenic mechanisms. To address this, integrating high-throughput sequencing technologies such as single-cell sequencing and spatial transcriptomics is crucial. These techniques can provide high-resolution insights into the cellular heterogeneity and spatial organization within bone tissue, enabling a deeper understanding of interactions between implants and osteogenesis-related pathways, which can guide the design of optimized smart implants for clinical applications.
-
(4)
Of particular note is that the achievement of comprehensive functions related to bone tissue regeneration, such as the additional antibacterial/bactericidal capacity, immunomodulation, and angiogenesis, is popularly being taken seriously by researchers because of the complications that actually appear in clinical applications of implants due to the functional deficiencies beyond osteogenesis. However, the spatiotemporal cooperations of these various functions, especially more functional categories of three or more, are the main research challenges and severely affect the further clinical use of this kind of smart bone scaffolds with multi-functions.
-
(5)
In recent years, the significant role of budding neurogenic bone regeneration is gradually recognized and valued by researchers due to the found of the important regulatory impact of nervous system on both skeletal regeneration and metabolism. Intraosseous nerves sense the changes in bone by transmitting mechanical or electrical signals to maintain its normal physiological activity. However, for successfully achieving this advanced function, a serious of involved mechanisms and manufacturing technologies of relevant implants still need to be further explored including the creation of a neurotrophic microenvironment conducive to osteogenesis, crosstalk behaviour between nerves and bone, neural regeneration-angiogenic crosstalk, and the intracellular regulatory mechanism of neural signals (e.g., neuropeptide) to osteogenic differentiation of stem cells.
-
(6)
For achieving multifunctional smart bone implants, the ultimate goal is to mimic living human bone tissue with almost identical structure and biological function, which involves the coordinated interaction of physiological functions throughout the body. Therefore, in addition to the exploration of crosstalk between skeletal and neural tissue, the interconnection mechanisms between bone regeneration and physiological activities such as the regulation of the cerebral cortex, immunoregulation, blood supply, and metabolism are poorly understood and still require a long way to clarify.
-
(7)
To investigate the osteogenic and multifunctional mechanisms of novel bone implants, the selection of appropriate animal models is essential. These models provide a valuable platform for studying implant-host interactions and assessing their multifunctional roles in bone therapy and tissue regeneration. These preclinical studies play a pivotal role in bridging the gap between experimental research and practical applications, ensuring the successful integration of smart implants into clinical practice.
-
(8)
The emergence of artificial intelligence (AI) and machine learning (ML) has introduced innovative strategies for the development of smart bone implants, enabling it possible for precise regulation of bone tissue regeneration. AI-driven deep learning models and ML-based finite element analysis significantly accelerate advancements in material design, personalized implant adaptation, and clinical outcome prediction. These approaches can enable high-throughput screening of biomaterial-cell interactions, optimize implant surface modifications for enhanced osteogenesis, and facilitate the generation of patient-specific implant models. Therefore, advancing AI- and ML-based predictive models for implant design and long-term performance evaluation will be essential for ensuring safer, more effective, and personalized treatment solutions in future development and application of the smart bone implants.
CRediT authorship contribution statement
Shishuo Li: Writing – review & editing, Writing – original draft, Validation, Investigation, Formal analysis, Data curation, Conceptualization. Zhentao Man: Writing – review & editing, Supervision, Funding acquisition, Formal analysis, Conceptualization. Kangqing Zuo: Supervision, Funding acquisition. Linbo Zhang: Data curation. Taixing Zhang: Formal analysis. Guiyong Xiao: Investigation, Formal analysis. Yupeng Lu: Funding acquisition, Data curation. Wei Li: Writing – review & editing, Validation, Supervision, Funding acquisition. Ningbo Li: Writing – review & editing, Writing – original draft, Validation, Funding acquisition, Conceptualization.
Ethics approval and consent to participate
This review manuscript does not involve animal experiments or clinical trials, so there is no ethics approval and consent to participate.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the financial support for this research from the Key Research and Development Program of Shandong Province (2021ZLGX01), Taishan Scholars Program for Young Experts of Shandong Province (tsqn202211348), Natural Science Foundation of Shandong Province (ZR2021MC176, ZR2023MH209, and ZR2024QE204)), China Postdoctoral Science Foundation (2024M751892), and Shandong Province Medical and Health Technology Project (202404070683).
Footnotes
Peer review under the responsibility of editorial board of Bioactive Materials.
Contributor Information
Yupeng Lu, Email: biosdu@sdu.edu.cn.
Wei Li, Email: liwei@sdfmu.edu.cn.
Ningbo Li, Email: liningbo@sdfmu.edu.cn.
References
- 1.Moriarty T.F., Metsemakers W.-J., Morgenstern M., Hofstee M.I., Vallejo Diaz A., Cassat J.E., Wildemann B., Depypere M., Schwarz E.M., Richards R.G. Fracture-related infection. Nat. Rev. Dis. Primers. 2022;8(1):67. doi: 10.1038/s41572-022-00396-0. [DOI] [PubMed] [Google Scholar]
- 2.Hoenig T., Ackerman K.E., Beck B.R., Bouxsein M.L., Burr D.B., Hollander K., Popp K.L., Rolvien T., Tenforde A.S., Warden S.J. Bone stress injuries. Nat. Rev. Dis. Primers. 2022;8(1):26. doi: 10.1038/s41572-022-00352-y. [DOI] [PubMed] [Google Scholar]
- 3.Wu A.-M., Bisignano C., James S.L., Abady G.G., Abedi A., Abu-Gharbieh E., Alhassan R.K., Alipour V., Arabloo J., Asaad M. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. The Lancet Healthy Longevity. 2021;2(9):e580–e592. doi: 10.1016/s2666-7568(21)00172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray C.J., Vos T., Lozano R., Naghavi M., Flaxman A.D., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 5.Challa S., Wu H.-H., Cunningham B.P., Liu M., Patel K., Shearer D.W., Morshed S., Miclau T. Orthopaedic trauma in the developing World: where are the gaps in research and what can be done? J. Orthop. Trauma. 2018;32:S43–S46. doi: 10.1097/BOT.0000000000001293. [DOI] [PubMed] [Google Scholar]
- 6.Chen B., Xiang H., Pan S., Yu L., Xu T., Chen Y. Advanced theragenerative biomaterials with therapeutic and regeneration multifunctionality. Adv. Funct. Mater. 2020;30(34) doi: 10.1002/adfm.202002621. [DOI] [Google Scholar]
- 7.Shen X., Zhang Y., Ma P., Sutrisno L., Luo Z., Hu Y., Yu Y., Tao B., Li C., Cai K. Fabrication of magnesium/zinc-metal organic framework on titanium implants to inhibit bacterial infection and promote bone regeneration. Biomaterials. 2019;212:1–16. doi: 10.1016/j.biomaterials.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Cui X., Xu L., Shan Y., Li J., Ji J., Wang E., Zhang B., Wen X., Bai Y., Luo D. Piezocatalytically-induced controllable mineralization scaffold with bone-like microenvironment to achieve endogenous bone regeneration. Science Bulletin. 2024 doi: 10.1016/j.scib.2024.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Olszta M.J., Cheng X., Jee S.S., Kumar R., Kim Y.-Y., Kaufman M.J., Douglas E.P., Gower L.B. Bone structure and formation: a new perspective. Mater. Sci. Eng. R Rep. 2007;58(3–5):77–116. doi: 10.1016/j.mser.2007.05.001. [DOI] [Google Scholar]
- 10.Turner C., Wang T., Burr D. Shear strength and fatigue properties of human cortical bone determined from pure shear tests. Calcif. Tissue Int. 2001;69(6):373–378. doi: 10.1007/s00223-001-1006-1. [DOI] [PubMed] [Google Scholar]
- 11.Mohanakrishnan V., Sivaraj K.K., Jeong H.-W., Bovay E., Dharmalingam B., Bixel M.G., Dinh V.V., Petkova M., Paredes Ugarte I., Kuo Y.-T. Specialized post-arterial capillaries facilitate adult bone remodelling. Nat. Cell Biol. 2024:1–15. doi: 10.1038/s41556-024-01545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun W., Ye B., Chen S., Zeng L., Lu H., Wan Y., Gao Q., Chen K., Qu Y., Wu B. Neuro–bone tissue engineering: emerging mechanisms, potential strategies, and current challenges. Bone Research. 2023;11(1):65. doi: 10.1038/s41413-023-00302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi C., Wu T., He Y., Zhang Y., Fu D. Recent advances in bone-targeted therapy. Pharmacol. Ther. 2020;207 doi: 10.1016/j.pharmthera.2020.107473. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S., Sun L., Zhang J., Liu S., Han J., Liu Y. Adverse impact of heavy metals on bone cells and bone metabolism dependently and independently through anemia. Adv. Sci. 2020;7(19) doi: 10.1002/advs.202000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darvell B.W. Bioactivity—symphony or cacophony? A personal view of a tangled field. Prosthesis. 2021;3(1):75–84. doi: 10.3390/prosthesis3010008. [DOI] [Google Scholar]
- 16.Mateu-Sanz M., Fuenteslópez C.V., Uribe-Gomez J., Haugen H.J., Pandit A., Ginebra M.-P., Hakimi O., Krallinger M., Samara A. Redefining biomaterial biocompatibility: challenges for artificial intelligence and text mining. Trends Biotechnol. 2024;42(4):402–417. doi: 10.1016/j.tibtech.2023.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Wan S., Chen Y., Huang C., Huang Z., Liang C., Deng X., Cheng Q. Scalable ultrastrong MXene films with superior osteogenesis. Nature. 2024;634(8036):1103–1110. doi: 10.1038/s41586-024-08067-8. [DOI] [PubMed] [Google Scholar]
- 18.Hasani-Sadrabadi M.M., Sarrion P., Pouraghaei S., Chau Y., Ansari S., Li S., Aghaloo T., Moshaverinia A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020;12(534) doi: 10.1126/scitranslmed.aay6853. [DOI] [PubMed] [Google Scholar]
- 19.Miron R.J., Bohner M., Zhang Y., Bosshardt D.D. Osteoinduction and osteoimmunology: emerging concepts. Periodontology. 2024;94(1):9–26. doi: 10.1111/prd.12519. 2000. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal R., García A.J. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 2015;94:53–62. doi: 10.1016/j.addr.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duda G.N., Geissler S., Checa S., Tsitsilonis S., Petersen A., Schmidt-Bleek K. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 2023;19(2):78–95. doi: 10.1038/s41584-022-00887-0. [DOI] [PubMed] [Google Scholar]
- 22.Wang L., You X., Zhang L., Zhang C., Zou W. Mechanical regulation of bone remodeling. Bone research. 2022;10(1):16. doi: 10.1038/s41413-022-00190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Zhang H., Hu Y., Jing Y., Geng Z., Su J. Bone repair biomaterials: a perspective from immunomodulation. Adv. Funct. Mater. 2022;32(51) doi: 10.1002/adfm.202208639. [DOI] [Google Scholar]
- 24.Li J., Zhang Z., Tang J., Hou Z., Li L., Li B. Emerging roles of nerve‐bone axis in modulating skeletal system. Med. Res. Rev. 2024;44(4):1867–1903. doi: 10.1002/med.22031. [DOI] [PubMed] [Google Scholar]
- 25.Chen X., Zhou J., Qian Y., Zhao L. Antibacterial coatings on orthopedic implants. Mater. Today Bio. 2023;19 doi: 10.1016/j.mtbio.2023.100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodges N.A., Sussman E.M., Stegemann J.P. Aseptic and septic prosthetic joint loosening: impact of biomaterial wear on immune cell function, inflammation, and infection. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121127. [DOI] [PubMed] [Google Scholar]
- 27.Wildemann B., Ignatius A., Leung F., Taitsman L.A., Smith R.M., Pesántez R., Stoddart M.J., Richards R.G., Jupiter J.B. Non-union bone fractures. Nat. Rev. Dis. Primers. 2021;7(1):57. doi: 10.1038/s41572-021-00289-8. [DOI] [PubMed] [Google Scholar]
- 28.Fang B., Qiu P., Xia C., Cai D., Zhao C., Chen Y., Wang H., Liu S., Cheng H., Tang Z. Extracellular matrix scaffold crosslinked with vancomycin for multifunctional antibacterial bone infection therapy. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120603. [DOI] [PubMed] [Google Scholar]
- 29.Wu S., Xu J., Zou L., Luo S., Yao R., Zheng B., Liang G., Wu D., Li Y. Long-lasting renewable antibacterial porous polymeric coatings enable titanium biomaterials to prevent and treat peri-implant infection. Nat. Commun. 2021;12(1):3303. doi: 10.1038/s41467-021-23069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng P., Cao T., Zhao X., Lu W., Miao S., Ning F., Wang D., Gao Y., Wang L., Pei G. Nidogen1-enriched extracellular vesicles accelerate angiogenesis and bone regeneration by targeting Myosin-10 to regulate endothelial cell adhesion. Bioact. Mater. 2022;12:185–197. doi: 10.1016/j.bioactmat.2021.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou H., Ye S., Xu M., Hao L., Chen J., Fang Z., Guo K., Chen Y., Wang L. Dynamic surface adapts to multiple service stages by orchestrating responsive polymers and functional peptides. Biomaterials. 2023;301 doi: 10.1016/j.biomaterials.2023.122200. [DOI] [PubMed] [Google Scholar]
- 32.Adebowale K., Liao R., Suja V.C., Kapate N., Lu A., Gao Y., Mitragotri S. Materials for cell surface engineering. Adv. Mater. 2024;36(43) doi: 10.1002/adma.202210059. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Y., Liang H., Liu X., Wu J., Yang C., Wong T.M., Kwan K.Y., Cheung K.M., Wu S., Yeung K.W. Regulation of macrophage polarization through surface topography design to facilitate implant-to-bone osteointegration. Sci. Adv. 2021;7(14) doi: 10.1126/sciadv.abf6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang T., Bai J., Lu M., Huang C., Geng D., Chen G., Wang L., Qi J., Cui W., Deng L. Engineering immunomodulatory and osteoinductive implant surfaces via mussel adhesion-mediated ion coordination and molecular clicking. Nat. Commun. 2022;13(1):1–17. doi: 10.1038/s41467-021-27816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao D.-W., Liu C., Zuo K.-Q., Su P., Li L.-B., Xiao G.-Y., Cheng L. Strontium-zinc phosphate chemical conversion coating improves the osseointegration of titanium implants by regulating macrophage polarization. Chem. Eng. J. 2021;408 doi: 10.1016/j.cej.2020.127362. [DOI] [Google Scholar]
- 36.Saul D., Khosla S. Fracture healing in the setting of Endocrine diseases, aging, and cellular senescence. Endocr. Rev. 2022;43(6):984–1002. doi: 10.1210/endrev/bnac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlson S.W., Sierra R.J., Trousdale R.T. Total Hip arthroplasty in patients with osteogenesis imperfecta. J. Arthroplast. 2020;35(8):2131–2135. doi: 10.1016/j.arth.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 38.Veth R., Schreuder B., van Beem H., Pruszczynski M., de Rooy J. Cryosurgery in aggressive, benign, and low-grade malignant bone tumours. Lancet Oncol. 2005;6(1):25–34. doi: 10.1016/S1470-2045(04)01710-3. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y., Luo D., Wang T. Hierarchical structures of bone and bioinspired bone tissue engineering. Small. 2016;12(34):4611–4632. doi: 10.1002/smll.201600626. [DOI] [PubMed] [Google Scholar]
- 40.Wu S., Liu X., Yeung K.W., Liu C., Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014;80:1–36. doi: 10.1016/j.mser.2014.04.001. [DOI] [Google Scholar]
- 41.Peng H., Hu B., Xie L.-Q., Su T., Li C.-J., Liu Y., Yang M., Xiao Y., Feng X., Zhou R. A mechanosensitive lipolytic factor in the bone marrow promotes osteogenesis and lymphopoiesis. Cell Metab. 2022;34(8):1168–1182. doi: 10.1016/j.cmet.2022.05.009. e1166. [DOI] [PubMed] [Google Scholar]
- 42.Li H., Wang H., Pan J., Li J., Zhang K., Duan W., Liang H., Chen K., Geng D., Shi Q. Nanoscaled bionic periosteum orchestrating the osteogenic microenvironment for sequential bone regeneration. ACS Appl. Mater. Interfaces. 2020;12(33):36823–36836. doi: 10.1021/acsami.0c06906. [DOI] [PubMed] [Google Scholar]
- 43.Komatsu N., Takayanagi H. Mechanisms of joint destruction in rheumatoid arthritis—immune cell–fibroblast–bone interactions. Nat. Rev. Rheumatol. 2022;18(7):415–429. doi: 10.1038/s41584-022-00793-5. [DOI] [PubMed] [Google Scholar]
- 44.Albrecht L.V., Pereira R.C., Salusky I.B. All the might of the osteocyte: emerging roles in chronic kidney disease. Kidney Int. 2023;104(5):910–915. doi: 10.1016/j.kint.2023.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Bassett J.D., Williams G.R. Role of thyroid hormones in skeletal development and bone maintenance. Endocr. Rev. 2016;37(2):135–187. doi: 10.1210/er.2015-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng Y.-F., Wang L., Zhang Y., Li X., Ma Z.-S., Zou J.-W., Lei W., Zhang Z.-Y. Effect of reactive oxygen species overproduction on osteogenesis of porous titanium implant in the present of diabetes mellitus. Biomaterials. 2013;34(9):2234–2243. doi: 10.1016/j.biomaterials.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Bai H., Zhao Y., Wang C., Wang Z., Wang J., Liu H., Feng Y., Lin Q., Li Z., Liu H. Enhanced osseointegration of three-dimensional supramolecular bioactive interface through osteoporotic microenvironment regulation. Theranostics. 2020;10(11):4779. doi: 10.7150/thno.43736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khosla S., Westendorf J.J., Oursler M.J. Building bone to reverse osteoporosis and repair fractures. J. Clin. Investig. 2008;118(2):421–428. doi: 10.1172/JCI33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cortet B. Bone repair in osteoporotic bone: postmenopausal and cortisone-induced osteoporosis. Osteoporos. Int. 2011;22(6):2007–2010. doi: 10.1007/s00198-011-1612-3. [DOI] [PubMed] [Google Scholar]
- 50.Yuan B., Wang L., Zhao R., Yang X., Yang X., Zhu X., Liu L., Zhang K., Song Y., Zhang X. A biomimetically hierarchical polyetherketoneketone scaffold for osteoporotic bone repair. Sci. Adv. 2020;6(50) doi: 10.1126/sciadv.abc4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Q.Y., Gong H.B., Jiang M.Y., Jin F., Wang G., Yan C.Y., Luo X., Sun W.Y., Ouyang S.H., Wu Y.P., Duan W.J., Liang L., Cao Y.F., Sun X.X., Liu M., Jiao G.L., Wang H.J., Hiroshi K., Wang X., He R.R., Li Y.F. Regulation of enzymatic lipid peroxidation in osteoblasts protects against postmenopausal osteoporosis. Nat. Commun. 2025;16(1):758. doi: 10.1038/s41467-025-55929-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meirow Y., Jovanovic M., Zur Y., Habib J., Colombo D.F., Twaik N., Ashkenazi-Preiser H., Ben-Meir K., Mikula I., Reuven O., Kariv G., Daniel L., Baraghithy S., Klein Y., Krijgsveld J., Levaot N., Baniyash M. Specific inflammatory osteoclast precursors induced during chronic inflammation give rise to highly active osteoclasts associated with inflammatory bone loss. Bone Research. 2022;10(1):36. doi: 10.1038/s41413-022-00206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L.-T., Chen L.-R., Chen K.-H. Hormone-related and drug-induced osteoporosis: a cellular and molecular overview. Int. J. Mol. Sci. 2023;24(6):5814. doi: 10.3390/ijms24065814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardy R.S., Zhou H., Seibel M.J., Cooper M.S. Glucocorticoids and bone: consequences of endogenous and exogenous excess and replacement therapy. Endocr. Rev. 2018;39(5):519–548. doi: 10.1210/er.2018-00097. [DOI] [PubMed] [Google Scholar]
- 55.Yu B., Wang C.-Y. Osteoporosis: the result of an ‘aged’bone microenvironment. Trends Mol. Med. 2016;22(8):641–644. doi: 10.1016/j.molmed.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schinke T., Schilling A.F., Baranowsky A., Seitz S., Marshall R.P., Linn T., Blaeker M., Huebner A.K., Schulz A., Simon R. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat. Med. 2009;15(6):674–681. doi: 10.1038/nm.1963. [DOI] [PubMed] [Google Scholar]
- 57.Zheng Y., Gao A., Bai J., Liao Q., Wu Y., Zhang W., Guan M., Tong L., Geng D., Zhao X. A programmed surface on polyetheretherketone for sequentially dictating osteoimmunomodulation and bone regeneration to achieve ameliorative osseointegration under osteoporotic conditions. Bioact. Mater. 2022;14:364–376. doi: 10.1016/j.bioactmat.2022.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen M., Wang D., Li M., He Y., He T., Chen M., Hu Y., Luo Z., Cai K. Nanocatalytic biofunctional MOF coating on titanium implants promotes osteoporotic bone regeneration through cooperative pro-osteoblastogenesis MSC reprogramming. ACS Nano. 2022;16(9):15397–15412. doi: 10.1021/acsnano.2c07200. [DOI] [PubMed] [Google Scholar]
- 59.Sheng N., Xing F., Wang J., Zhang Q.-Y., Nie R., Li-Ling J., Duan X., Xie H.-Q. Recent progress in bone-repair strategies in diabetic conditions. Mater. Today Bio. 2023;23 doi: 10.1016/j.mtbio.2023.100835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crane J.L., Zhao L., Frye J.S., Xian L., Qiu T., Cao X. IGF-1 signaling is essential for differentiation of mesenchymal stem cells for peak bone mass. Bone Research. 2013;1(1):186–194. doi: 10.4248/BR201302007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shanbhogue V.V., Hansen S., Frost M., Brixen K., Hermann A.P. Bone disease in diabetes: another manifestation of microvascular disease? Lancet Diabetes Endocrinol. 2017;5(10):827–838. doi: 10.1016/S2213-8587(17)30134-1. [DOI] [PubMed] [Google Scholar]
- 62.Khosla S., Samakkarnthai P., Monroe D.G., Farr J.N. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021;17(11):685–697. doi: 10.1038/s41574-021-00555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Napoli N., Chandran M., Pierroz D.D., Abrahamsen B., Schwartz A.V., Ferrari S.L., Bone I., Group D.W. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017;13(4):208–219. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 64.Mangialardi G., Ferland-McCollough D., Maselli D., Santopaolo M., Cordaro A., Spinetti G., Sambataro M., Sullivan N., Blom A., Madeddu P. Bone marrow pericyte dysfunction in individuals with type 2 diabetes. Diabetologia. 2019;62:1275–1290. doi: 10.1007/s00125-019-4865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melis S., Trompet D., Chagin A.S., Maes C. Skeletal stem and progenitor cells in bone physiology, ageing and disease. Nat. Rev. Endocrinol. 2024:1–19. doi: 10.1038/s41574-024-01039-y. [DOI] [PubMed] [Google Scholar]
- 66.Stewart P., Bjarnsholt T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin. Microbiol. Infection. 2020;26(8):1034–1038. doi: 10.1016/j.cmi.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 67.Kapadia B.H., Berg R.A., Daley J.A., Fritz J., Bhave A., Mont M.A. Periprosthetic joint infection. Lancet. 2016;387(10016):386–394. doi: 10.1016/S0140-6736(14)61798-0. [DOI] [PubMed] [Google Scholar]
- 68.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007;7(4):292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 69.Smolen J.S., Aletaha D., Barton A., Burmester G.R., Emery P., Firestein G.S., Kavanaugh A., McInnes I.B., Solomon D.H., Strand V., Yamamoto K. Rheumatoid arthritis. Nat. Rev. Dis. Primers. 2018;4(1) doi: 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 70.McInnes I.B., Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007;7(6):429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 71.Chen Z., Bozec A., Ramming A., Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019;15(1):9–17. doi: 10.1038/s41584-018-0109-2. [DOI] [PubMed] [Google Scholar]
- 72.Germar K., Fehres C.M., Scherer H.U., van Uden N., Pollastro S., Yeremenko N., Hansson M., Kerkman P.F., van der Voort E.I., Reed E. Generation and characterization of anti–citrullinated protein antibody–producing B cell clones from rheumatoid arthritis patients. Arthritis Rheumatol. 2019;71(3):340–350. doi: 10.1002/art.40739. [DOI] [PubMed] [Google Scholar]
- 73.Conigliaro P., Chimenti M., Triggianese P., Sunzini F., Novelli L., Perricone C., Perricone R. Autoantibodies in inflammatory arthritis. Autoimmun. Rev. 2016;15(7):673–683. doi: 10.1016/j.autrev.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 74.Rombouts Y., Ewing E., Van De Stadt L.A., Selman M.H., Trouw L.A., Deelder A.M., Huizinga T.W., Wuhrer M., Van Schaardenburg D., Toes R.E. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann. Rheum. Dis. 2015;74(1):234–241. doi: 10.1136/annrheumdis-2013-203565. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y., Krishnamurthy A., Hensvold A., Joshua V., Sun M., Engstrom M., Wähämaa H., Malmström V., Jopling L., Rethi B. AB0078 Role of IL-8 and its receptor in anti-citrullinated protein antibody mediated osteoclastogenesis in RA. Ann. Rheum. Dis. 2016;75:923. doi: 10.1136/annrheumdis-2016-eular.5380. [DOI] [Google Scholar]
- 76.Maeda K., Kobayashi Y., Udagawa N., Uehara S., Ishihara A., Mizoguchi T., Kikuchi Y., Takada I., Kato S., Kani S. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat. Med. 2012;18(3):405–412. doi: 10.1038/nm.2653. [DOI] [PubMed] [Google Scholar]
- 77.Sun W., Meednu N., Rosenberg A., Rangel-Moreno J., Wang V., Glanzman J., Owen T., Zhou X., Zhang H., Boyce B.F. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat. Commun. 2018;9(1):5127. doi: 10.1038/s41467-018-07626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kachler K., Andreev D., Thapa S., Royzman D., Gießl A., Karuppusamy S., Perez M.L., Liu M., Hofmann J., Gessner A. Acod1-mediated inhibition of aerobic glycolysis suppresses osteoclast differentiation and attenuates bone erosion in arthritis. Ann. Rheum. Dis. 2024;83(12):1691–1706. doi: 10.1136/ard-2023-224774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J., Wang P., Xie Z., Wang S., Cen S., Li M., Liu W., Tang S., Ye G., Zheng G., Su H., Ma M., Wu X., Wu Y., Shen H. TRAF4 positively regulates the osteogenic differentiation of mesenchymal stem cells by acting as an E3 ubiquitin ligase to degrade Smurf2. Cell Death Differ. 2019;26(12):2652–2666. doi: 10.1038/s41418-019-0328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lowery J.W., Rosen V. The BMP pathway and its inhibitors in the skeleton. Physiol. Rev. 2018;98(4):2431–2452. doi: 10.1152/physrev.00028.2017. [DOI] [PubMed] [Google Scholar]
- 81.Appelman-Dijkstra N.M., Papapoulos S.E. Clinical advantages and disadvantages of anabolic bone therapies targeting the WNT pathway. Nat. Rev. Endocrinol. 2018;14(10):605–623. doi: 10.1038/s41574-018-0087-0. [DOI] [PubMed] [Google Scholar]
- 82.Klar R.M., Duarte R., Dix-Peek T., Ripamonti U. The induction of bone formation by the recombinant human transforming growth factor-beta3. Biomaterials. 2014;35(9):2773–2788. doi: 10.1016/j.biomaterials.2013.12.062. [DOI] [PubMed] [Google Scholar]
- 83.Wang Z., Liu Q., Liu C., Tan W., Tang M., Zhou X., Sun T., Deng Y. Mg(2+) in beta-TCP/Mg-Zn composite enhances the differentiation of human bone marrow stromal cells into osteoblasts through MAPK-regulated Runx2/Osx. J. Cell. Physiol. 2020;235(6):5182–5191. doi: 10.1002/jcp.29395. [DOI] [PubMed] [Google Scholar]
- 84.Shah M., Kola B., Bataveljic A., Arnett T., Viollet B., Saxon L., Korbonits M., Chenu C. AMP-activated protein kinase (AMPK) activation regulates in vitro bone formation and bone mass. Bone. 2010;47(2):309–319. doi: 10.1016/j.bone.2010.04.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Armiento A.R., Hatt L.P., Sanchez Rosenberg G., Thompson K., Stoddart M.J. Functional biomaterials for bone regeneration: a lesson in complex biology. Adv. Funct. Mater. 2020;30(44) doi: 10.1002/adfm.201909874. [DOI] [Google Scholar]
- 86.Zhang K., Wang S., Zhou C., Cheng L., Gao X., Xie X., Sun J., Wang H., Weir M.D., Reynolds M.A., Zhang N., Bai Y., Xu H.H.K. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Research. 2018;6(1):31. doi: 10.1038/s41413-018-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Z., Xiang S., Lin Z., Li E.N., Yagi H., Cao G., Yocum L., Li, Hao T., Bruce K.K., Fritch M.R., Hu H., Wang B., Alexander P.G., Khor K.A., Tuan R.S., Lin H. Graphene oxide-functionalized nanocomposites promote osteogenesis of human mesenchymal stem cells via enhancement of BMP-SMAD1/5 signaling pathway. Biomaterials. 2021;277 doi: 10.1016/j.biomaterials.2021.121082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li L., Zhang X., Zhou J., Zhang L., Xue J., Tao W. Non‐invasive thermal therapy for tissue engineering and regenerative medicine. Small. 2022;18(36) doi: 10.1002/smll.202107705. [DOI] [PubMed] [Google Scholar]
- 89.Ma L., Feng X., Liang H., Wang K., Song Y., Tan L., Wang B., Luo R., Liao Z., Li G., Liu X., Wu S., Yang C. A novel photothermally controlled multifunctional scaffold for clinical treatment of osteosarcoma and tissue regeneration. Mater. Today. 2020;36:48–62. doi: 10.1016/j.mattod.2019.12.005. [DOI] [Google Scholar]
- 90.Shan H., Zhou X., Tian B., Zhou C., Gao X., Bai C., Shan B., Zhang Y., Sun S., Sun D., Fan Q., Zhou X., Wang C., Bai J. Gold nanorods modified by endogenous protein with light-irradiation enhance bone repair via multiple osteogenic signal pathways. Biomaterials. 2022;284 doi: 10.1016/j.biomaterials.2022.121482. [DOI] [PubMed] [Google Scholar]
- 91.Metavarayuth K., Maturavongsadit P., Chen X., Sitasuwan P., Lu L., Su J., Wang Q. Nanotopographical cues mediate osteogenesis of stem cells on virus substrates through BMP-2 intermediate. Nano Lett. 2019;19(12):8372–8380. doi: 10.1021/acs.nanolett.9b02001. [DOI] [PubMed] [Google Scholar]
- 92.Wu L., Feyerabend F., Schilling A.F., Willumeit-Romer R., Luthringer B.J.C. Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater. 2015;27:294–304. doi: 10.1016/j.actbio.2015.08.042. [DOI] [PubMed] [Google Scholar]
- 93.Chen X., Tan B., Wang S., Tang R., Bao Z., Chen G., Chen S., Tang W., Wang Z., Long C., Lu W.W., Yang D., Bian L., Peng S. Rationally designed protein cross-linked hydrogel for bone regeneration via synergistic release of magnesium and zinc ions. Biomaterials. 2021;274 doi: 10.1016/j.biomaterials.2021.120895. [DOI] [PubMed] [Google Scholar]
- 94.Yao M., Cheng S., Zhong G., Zhou J., Shao H., Ma L., Du C., Peng F., Zhang Y. Enhanced osteogenesis of titanium with nano-Mg(OH)2 film and a mechanism study via whole genome expression analysis. Bioact. Mater. 2021;6(9):2729–2741. doi: 10.1016/j.bioactmat.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fowler T.W., Mitchell T.L., Janda C.Y., Xie L., Tu S., Chen H., Zhang H., Ye J., Ouyang B., Yuan T.Z., Lee S.J., Newman M., Tripuraneni N., Rego E.S., Mutha D., Dilip A., Vuppalapaty M., Baribault H., Yeh W.C., Li Y. Development of selective bispecific Wnt mimetics for bone loss and repair. Nat. Commun. 2021;12(1):3247. doi: 10.1038/s41467-021-23374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhao X., Wang S., Wang F., Zhu Y., Gu R., Yang F., Xu Y., Xia D., Liu Y. 3D-printed Mg-1Ca/polycaprolactone composite scaffolds with promoted bone regeneration. J. Magnesium Alloys. 2024;12(3):966–979. doi: 10.1016/j.jma.2022.07.002. [DOI] [Google Scholar]
- 97.Li R., Lin S., Zhu M., Deng Y., Chen X., Wei K., Xu J., Li G., Bian L. Synthetic presentation of noncanonical Wnt5a motif promotes mechanosensing-dependent differentiation of stem cells and regeneration. Sci. Adv. 2019;5(10) doi: 10.1126/sciadv.aaw3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okuchi Y., Reeves J., Ng S.S., Doro D.H., Junyent S., Liu K.J., El Haj A.J., Habib S.J. Wnt-modified materials mediate asymmetric stem cell division to direct human osteogenic tissue formation for bone repair. Nat. Mater. 2021;20(1):108–118. doi: 10.1038/s41563-020-0786-5. [DOI] [PubMed] [Google Scholar]
- 99.Salhotra A., Shah H.N., Levi B., Longaker M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020;21(11):696–711. doi: 10.1038/s41580-020-00279-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kassel K.M., Owens A.P., 3rd, Rockwell C.E., Sullivan B.P., Wang R., Tawfik O., Li G., Guo G.L., Mackman N., Luyendyk J.P. Protease-activated receptor 1 and hematopoietic cell tissue factor are required for hepatic steatosis in mice fed a Western diet. Am. J. Pathol. 2011;179(5):2278–2289. doi: 10.1016/j.ajpath.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li J., Deng C., Liang W., Kang F., Bai Y., Ma B., Wu C., Dong S. Mn-containing bioceramics inhibit osteoclastogenesis and promote osteoporotic bone regeneration via scavenging ROS. Bioact. Mater. 2021;6(11):3839–3850. doi: 10.1016/j.bioactmat.2021.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He Y., Li Z., Ding X., Xu B., Wang J., Li Y., Chen F., Meng F., Song W., Zhang Y. Nanoporous titanium implant surface promotes osteogenesis by suppressing osteoclastogenesis via integrin beta1/FAKpY397/MAPK pathway. Bioact. Mater. 2022;8:109–123. doi: 10.1016/j.bioactmat.2021.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Z., Wang H., Zhang K., Yang B., Xie X., Yang Z., Kong L., Shi P., Zhang Y., Ho Y.P., Zhang Z.Y., Li G., Bian L. Bisphosphonate-based hydrogel mediates biomimetic negative feedback regulation of osteoclastic activity to promote bone regeneration. Bioact. Mater. 2022;13:9–22. doi: 10.1016/j.bioactmat.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Minoshima M., Kikuta J., Omori Y., Seno S., Suehara R., Maeda H., Matsuda H., Ishii M., Kikuchi K. In vivo multicolor imaging with fluorescent probes revealed the dynamics and function of osteoclast proton pumps. ACS Cent. Sci. 2019;5(6):1059–1066. doi: 10.1021/acscentsci.9b00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakashima T., Takayanagi H. New regulation mechanisms of osteoclast differentiation. Ann. N. Y. Acad. Sci. 2011;1240(1):E13–E18. doi: 10.1111/j.1749-6632.2011.06373.x. [DOI] [PubMed] [Google Scholar]
- 106.Ren X., Zhou Q., Foulad D., Tiffany A.S., Dewey M.J., Bischoff D., Miller T.A., Reid R.R., He T.C., Yamaguchi D.T., Harley B.A.C., Lee J.C. Osteoprotegerin reduces osteoclast resorption activity without affecting osteogenesis on nanoparticulate mineralized collagen scaffolds. Sci. Adv. 2019;5(6):eaaw4991. doi: 10.1126/sciadv.aaw4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Histing T., Anton C., Scheuer C., Garcia P., Holstein J.H., Klein M., Matthys R., Pohlemann T., Menger M.D. Melatonin impairs fracture healing by suppressing RANKL-mediated bone remodeling. J. Surg. Res. 2012;173(1):83–90. doi: 10.1016/j.jss.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 108.Wang X., Yu Y., Ji L., Geng Z., Wang J., Liu C. Calcium phosphate-based materials regulate osteoclast-mediated osseointegration. Bioact. Mater. 2021;6(12):4517–4530. doi: 10.1016/j.bioactmat.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun K.Y., Wu Y., Xu J., Xiong W., Xu W., Li J., Sun Z., Lv Z., Wu X.S., Jiang Q., Cai H.L., Shi D. Niobium carbide (MXene) reduces UHMWPE particle-induced osteolysis. Bioact. Mater. 2022;8:435–448. doi: 10.1016/j.bioactmat.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li S., Lu X., Chai Q., Huang B., Dai S., Wang P., Liu J., Zhao Z., Li X., Liu B. Engineered niobium carbide MXenzyme-integrated self-adaptive coatings inhibiting periprosthetic osteolysis by orchestrating osteogenesis–osteoclastogenesis balance. ACS Appl. Mater. Interfaces. 2024;16(23):29805–29822. doi: 10.1021/acsami.4c04122. [DOI] [PubMed] [Google Scholar]
- 111.Kim H., Lee K., Kim J.M., Kim M.Y., Kim J.R., Lee H.W., Chung Y.W., Shin H.I., Kim T., Park E.S., Rho J., Lee S.H., Kim N., Lee S.Y., Choi Y., Jeong D. Selenoprotein W ensures physiological bone remodeling by preventing hyperactivity of osteoclasts. Nat. Commun. 2021;12(1):2258. doi: 10.1038/s41467-021-22565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jaber F.A., Khan N.M., Ansari M.Y., Al-Adlaan A.A., Hussein N.J., Safadi F.F. Autophagy plays an essential role in bone homeostasis. J. Cell. Physiol. 2019;234(8):12105–12115. doi: 10.1002/jcp.27071. [DOI] [PubMed] [Google Scholar]
- 113.Yin X., Zhou C., Li J., Liu R., Shi B., Yuan Q., Zou S. Autophagy in bone homeostasis and the onset of osteoporosis. Bone Research. 2019;7:28. doi: 10.1038/s41413-019-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nollet M., Santucci-Darmanin S., Breuil V., Al-Sahlanee R., Cros C., Topi M., Momier D., Samson M., Pagnotta S., Cailleteau L., Battaglia S., Farlay D., Dacquin R., Barois N., Jurdic P., Boivin G., Heymann D., Lafont F., Lu S.S., Dempster D.W., Carle G.F., Pierrefite-Carle V. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy. 2014;10(11):1965–1977. doi: 10.4161/auto.36182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang S., Xie Y., Yan F., Zhang Y., Yang Z., Chen Z., Zhao Y., Huang Z., Cai L., Deng Z. Negative pressure wound therapy improves bone regeneration by promoting osteogenic differentiation via the AMPK-ULK1-autophagy axis. Autophagy. 2021;18(9):1–17. doi: 10.1080/15548627.2021.2016231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qi C., Lin J., Fu L.H., Huang P. Calcium-based biomaterials for diagnosis, treatment, and theranostics. Chem. Soc. Rev. 2018;47(2):357–403. doi: 10.1039/c6cs00746e. [DOI] [PubMed] [Google Scholar]
- 117.Hu L., Chen W., Qian A., Li Y.-P. Wnt/β-catenin signaling components and mechanisms in bone formation, homeostasis, and disease. Bone Research. 2024;12(1):39. doi: 10.1038/s41413-024-00342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu W., Wang T., Zhao X., Dan X., Lu W.W., Pan H. Akermanite used as an alkaline biodegradable implants for the treatment of osteoporotic bone defect. Bioact. Mater. 2016;1(2):151–159. doi: 10.1016/j.bioactmat.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu W., Dan X., Lu W.W., Zhao X., Ruan C., Wang T., Cui X., Zhai X., Ma Y., Wang D., Huang W., Pan H. Spatial distribution of biomaterial microenvironment pH and its modulatory effect on osteoclasts at the early stage of bone defect regeneration. ACS Appl. Mater. Interfaces. 2019;11(9):9557–9572. doi: 10.1021/acsami.8b20580. [DOI] [PubMed] [Google Scholar]
- 120.Chen M., Hu Y., Hou Y., Li M., Tan L., Chen M., Geng W., Tao B., Jiang H., Luo Z., Cai K. Magnesium/gallium-layered nanosheets on titanium implants mediate osteogenic differentiation of MSCs and osseointegration under osteoporotic condition. Chem. Eng. J. 2022;427 doi: 10.1016/j.cej.2021.130982. [DOI] [Google Scholar]
- 121.Li L., Yang S., Xu L., Li Y., Fu Y., Zhang H., Song J. Nanotopography on titanium promotes osteogenesis via autophagy-mediated signaling between YAP and beta-catenin. Acta Biomater. 2019;96:674–685. doi: 10.1016/j.actbio.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 122.Wang H., Ma Y., Li J., Zhou C., Xu A., Xu Y., He F. Modulating autophagy by strontium-doped micro/nano rough titanium surface for promotion of osteogenesis and inhibition of osteoclastogenesis. Colloids Surf. B Biointerfaces. 2022;210 doi: 10.1016/j.colsurfb.2021.112246. [DOI] [PubMed] [Google Scholar]
- 123.Pan H., Wang H., Yu J., Huang X., Hao Y., Zhang C., Ji W., Yang M., Gong X., Wu X., Chang J. Near-infrared light remotely up-regulate autophagy with spatiotemporal precision via upconversion optogenetic nanosystem. Biomaterials. 2019;199:22–31. doi: 10.1016/j.biomaterials.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 124.Dohan Ehrenfest D.M., Coelho P.G., Kang B.S., Sul Y.T., Albrektsson T. Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Biotechnol. 2010;28(4):198–206. doi: 10.1016/j.tibtech.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 125.Liang Y., Qin H., Mehra N., Zhu J., Yang Z., Doll G.L., Ye C., Dong Y. Controllable hierarchical micro/nano patterns on biomaterial surfaces fabricated by ultrasonic nanocrystalline surface modification. Mater. Des. 2018;137:325–334. doi: 10.1016/j.matdes.2017.10.041. [DOI] [Google Scholar]
- 126.Zhang Z., Xu R., Yang Y., Liang C., Yu X., Liu Y., Wang T., Yu Y., Deng F. Micro/nano-textured hierarchical titanium topography promotes exosome biogenesis and secretion to improve osseointegration. J. Nanobiotechnol. 2021;19(1):78. doi: 10.1186/s12951-021-00826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang N., Liu X., Tang Z., Wei X., Dong H., Liu Y., Wu H., Wu Z., Li X., Ma X., Guo Z. Increased BMSC exosomal miR-140-3p alleviates bone degradation and promotes bone restoration by targeting Plxnb1 in diabetic rats. J. Nanobiotechnol. 2022;20(1):97. doi: 10.1186/s12951-022-01267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang J., Liu X., Li H., Chen C., Hu B., Niu X., Li Q., Zhao B., Xie Z., Wang Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016;7(1):136. doi: 10.1186/s13287-016-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ma L., Li G., Lei J., Song Y., Feng X., Tan L., Luo R., Liao Z., Shi Y., Zhang W., Liu X., Sheng W., Wu S., Yang C. Nanotopography sequentially mediates human mesenchymal stem cell-derived small extracellular vesicles for enhancing osteogenesis. ACS Nano. 2021;16(1):415–430. doi: 10.1021/acsnano.1c07150. [DOI] [PubMed] [Google Scholar]
- 130.Zhai M., Zhu Y., Yang M., Mao C. Human mesenchymal stem cell derived exosomes enhance cell-free bone regeneration by altering their miRNAs profiles. Adv. Sci. 2020;7(19) doi: 10.1002/advs.202001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carthew J., Abdelmaksoud H.H., Hodgson-Garms M., Aslanoglou S., Ghavamian S., Elnathan R., Spatz J.P., Brugger J., Thissen H., Voelcker N.H., Cadarso V.J., Frith J.E. Precision surface microtopography regulates cell fate via changes to actomyosin contractility and nuclear architecture. Adv. Sci. 2021;8(6) doi: 10.1002/advs.202003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang X., Agrawal V., Dunton C.L., Liu Y., Virk R.K.A., Patel P.A., Carter L., Pujadas E.M., Li Y., Jain S., Wang H., Ni N., Tsai H.M., Rivera-Bolanos N., Frederick J., Roth E., Bleher R., Duan C., Ntziachristos P., He T.C., Reid R.R., Jiang B., Subramanian H., Backman V., Ameer G.A. Chromatin reprogramming and bone regeneration in vitro and in vivo via the microtopography-induced constriction of cell nuclei. Nat. Biomed. Eng. 2023;7(11):1514–1529. doi: 10.1038/s41551-023-01053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sabino R.M., Rau J.V., De Bonis A., De Stefanis A., Curcio M., Teghil R., Popat K.C. Manganese-containing bioactive glass enhances osteogenic activity of TiO2 nanotube arrays. Appl. Surf. Sci. 2021;570 doi: 10.1016/j.apsusc.2021.151163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bai L., Zhao Y., Chen P., Zhang X., Huang X., Du Z., Crawford R., Yao X., Tang B., Hang R., Xiao Y. Targeting early healing phase with titania nanotube arrays on tunable diameters to accelerate bone regeneration and osseointegration. Small. 2021;17(4) doi: 10.1002/smll.202006287. [DOI] [PubMed] [Google Scholar]
- 135.Mei P., Jiang S., Mao L., Zhou Y., Gu K., Zhang C., Wang X., Lin K., Zhao C., Zhu M. In situ construction of flower-like nanostructured calcium silicate bioceramics for enhancing bone regeneration mediated via FAK/p38 signaling pathway. J. Nanobiotechnol. 2022;20(1):162. doi: 10.1186/s12951-022-01361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li M., Ma H., Han F., Zhai D., Zhang B., Sun Y., Li T., Chen L., Wu C. Microbially catalyzed biomaterials for bone regeneration. Adv. Mater. 2021;33(49) doi: 10.1002/adma.202104829. [DOI] [PubMed] [Google Scholar]
- 137.Li Y., Liu Y., Chen H., Zhang A., Zhang Y., Zhang J., Chen B., Han Q., Wang J. From clinic to lab: advances in porous titanium-based orthopedic implant research. J. Mater. Res. Technol. 2024;30:3780–3806. doi: 10.1016/j.jmrt.2024.04.136. [DOI] [Google Scholar]
- 138.Xie K., Wang L., Guo Y., Zhao S., Yang Y., Dong D., Ding W., Dai K., Gong W., Yuan G. Effectiveness and safety of biodegradable Mg-Nd-Zn-Zr alloy screws for the treatment of medial malleolar fractures. Journal of Orthopaedic Translation. 2021;27:96–100. doi: 10.1016/j.jot.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018;16(7):397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- 140.Le P.H., Linklater D.P., Medina A.A., MacLaughlin S., Crawford R.J., Ivanova E.P. Impact of multiscale surface topography characteristics on Candida albicans biofilm formation: from cell repellence to fungicidal activity. Acta Biomater. 2024;177:20–36. doi: 10.1016/j.actbio.2024.02.006. [DOI] [PubMed] [Google Scholar]
- 141.Yuan Y., Xu Y., Mao Y., Liu H., Ou M., Lin Z., Zhao R., Long H., Cheng L., Sun B., Zhao S., Zeng M., Lu B., Lu H., Zhu Y., Chen C. Three birds, one stone: an osteo-microenvironment stage-regulative scaffold for bone defect repair through modulating early osteo-immunomodulation, middle neovascularization, and later osteogenesis. Adv. Sci. 2024;11(6) doi: 10.1002/advs.202306428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Azad M.A., Patel R. Practical guidance for clinical microbiology laboratories: microbiologic diagnosis of implant-associated infections. Clin. Microbiol. Rev. 2024;37(2) doi: 10.1128/cmr.00104-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gristina A.G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 144.Bakhshandeh S., Gorgin Karaji Z., Lietaert K., Fluit A.C., Boel C.H.E., Vogely H.C., Vermonden T., Hennink W.E., Weinans H., Zadpoor A.A., Amin Yavari S. Simultaneous delivery of multiple antibacterial agents from additively manufactured porous biomaterials to fully eradicate planktonic and adherent Staphylococcus aureus. ACS Appl. Mater. Interfaces. 2017;9(31):25691–25699. doi: 10.1021/acsami.7b04950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gao S., Li Y., Xiao D., Zhou M., Cai X., Lin Y. Tetrahedral framework nucleic acids induce immune tolerance and prevent the onset of type 1 diabetes. Nano Lett. 2021;21(10):4437–4446. doi: 10.1021/acs.nanolett.1c01131. [DOI] [PubMed] [Google Scholar]
- 146.Zhang T., Tian T., Lin Y. Functionalizing framework nucleic‐acid‐based nanostructures for biomedical application. Adv. Mater. 2021;34(46) doi: 10.1002/adma.202107820. [DOI] [PubMed] [Google Scholar]
- 147.Li J., Lai Y., Li M., Chen X., Zhou M., Wang W., Li J., Cui W., Zhang G., Wang K., Liu L., Lin Y. Repair of infected bone defect with Clindamycin-Tetrahedral DNA nanostructure Complex-loaded 3D bioprinted hybrid scaffold. Chem. Eng. J. 2022;435 doi: 10.1016/j.cej.2022.134855. [DOI] [Google Scholar]
- 148.Abbas M., Ovais M., Atiq A., Ansari T.M., Xing R., Spruijt E., Yan X. Tailoring supramolecular short peptide nanomaterials for antibacterial applications. Coord. Chem. Rev. 2022;460 doi: 10.1016/j.ccr.2022.214481. [DOI] [Google Scholar]
- 149.Li N., Bai J., Wang W., Liang X., Zhang W., Li W., Lu L., Xiao L., Xu Y., Wang Z., Zhu C., Zhou J., Geng D. Facile and versatile surface functional polyetheretherketone with enhanced bacteriostasis and osseointegrative capability for implant application. ACS Appl. Mater. Interfaces. 2021;13(50):59731–59746. doi: 10.1021/acsami.1c19834. [DOI] [PubMed] [Google Scholar]
- 150.Mo S., Tang K., Liao Q., Xie L., Wu Y., Wang G., Ruan Q., Gao A., Lv Y., Cai K. Tuning the arrangement of lamellar nanostructures: achieving the dual function of physically killing bacteria and promoting osteogenesis. Mater. Horiz. 2023;10(3):881–888. doi: 10.1039/D2MH01147F. [DOI] [PubMed] [Google Scholar]
- 151.Ye J., Li B., Zheng Y., Wu S., Chen D., Han Y. Eco-friendly bacteria-killing by nanorods through mechano-puncture with top selectivity. Bioact. Mater. 2022;15:173–184. doi: 10.1016/j.bioactmat.2021.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Muiznieks L.D., Keeley F.W. Molecular assembly and mechanical properties of the extracellular matrix: a fibrous protein perspective. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2013;1832(7):866–875. doi: 10.1016/j.bbadis.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 153.Nikolaev N.I., Muller T., Williams D.J., Liu Y. Changes in the stiffness of human mesenchymal stem cells with the progress of cell death as measured by atomic force microscopy. J. Biomech. 2014;47(3):625–630. doi: 10.1016/j.jbiomech.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 154.Yu Q., Cho J., Shivapooja P., Ista L.K., Lopez G.P. Nanopatterned smart polymer surfaces for controlled attachment, killing, and release of bacteria. ACS Appl. Mater. Interfaces. 2013;5(19):9295–9304. doi: 10.1021/am4022279. [DOI] [PubMed] [Google Scholar]
- 155.Zhang X., Zhang G., Chai M., Yao X., Chen W., Chu P.K. Synergistic antibacterial activity of physical-chemical multi-mechanism by TiO2 nanorod arrays for safe biofilm eradication on implant. Bioact. Mater. 2021;6(1):12–25. doi: 10.1016/j.bioactmat.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sirelkhatim A., Mahmud S., Seeni A., Kaus N.H.M., Ann L.C., Bakhori S.K.M., Hasan H., Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015;7(3):219–242. doi: 10.1007/s40820-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li J., Tan L., Liu X., Cui Z., Yang X., Yeung K.W.K., Chu P.K., Wu S. Balancing bacteria-osteoblast competition through selective physical puncture and biofunctionalization of ZnO/Polydopamine/Arginine-Glycine-Aspartic acid-cysteine nanorods. ACS Nano. 2017;11(11):11250–11263. doi: 10.1021/acsnano.7b05620. [DOI] [PubMed] [Google Scholar]
- 158.Ye J., Li B., Li M., Zheng Y., Wu S., Han Y. Formation of a ZnO nanorods-patterned coating with strong bactericidal capability and quantitative evaluation of the contribution of nanorods-derived puncture and ROS-derived killing. Bioact. Mater. 2022;11:181–191. doi: 10.1016/j.bioactmat.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao F., Gao A., Liao Q., Li Y., Ullah I., Zhao Y., Ren X., Tong L., Li X., Zheng Y. Balancing the anti‐bacterial and pro‐osteogenic properties of Ti‐based implants by partial conversion of ZnO nanorods into hybrid zinc phosphate nanostructures. Adv. Funct. Mater. 2024;34(17) doi: 10.1002/adfm.202311812. [DOI] [Google Scholar]
- 160.Zhao C., Zhou L., Chiao M., Yang W. Antibacterial hydrogel coating: strategies in surface chemistry. Adv. Colloid Interface Sci. 2020;285 doi: 10.1016/j.cis.2020.102280. [DOI] [PubMed] [Google Scholar]
- 161.Qu X., Yang H., Jia B., Wang M., Yue B., Zheng Y., Dai K. Zinc alloy-based bone internal fixation screw with antibacterial and anti-osteolytic properties. Bioact. Mater. 2021;6(12):4607–4624. doi: 10.1016/j.bioactmat.2021.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Birsoy K., Wang T., Chen W.W., Freinkman E., Abu-Remaileh M., Sabatini D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 2015;162(3):540–551. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shi L., Dong H., Reguera G., Beyenal H., Lu A., Liu J., Yu H.-Q., Fredrickson J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016;14(10):651–662. doi: 10.1038/nrmicro.2016.93. [DOI] [PubMed] [Google Scholar]
- 164.Fu J., Zhu W., Liu X., Liang C., Zheng Y., Li Z., Liang Y., Zheng D., Zhu S., Cui Z., Wu S. Self-activating anti-infection implant. Nat. Commun. 2021;12(1):6907. doi: 10.1038/s41467-021-27217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zheng S., Bawazir M., Dhall A., Kim H.E., He L., Heo J., Hwang G. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.643722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Halder S., Yadav K.K., Sarkar R., Mukherjee S., Saha P., Haldar S., Karmakar S., Sen T. Alteration of Zeta potential and membrane permeability in bacteria: a study with cationic agents. SpringerPlus. 2015;4:672. doi: 10.1186/s40064-015-1476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shi R., Zhang J., Tian J., Zhao C., Li Z., Zhang Y., Li Y., Wu C., Tian W., Li Z. An effective self-powered strategy to endow titanium implant surface with associated activity of anti-biofilm and osteogenesis. Nano Energy. 2020;77 doi: 10.1016/j.nanoen.2020.105201. [DOI] [Google Scholar]
- 168.Tan J., Wang D., Cao H., Qiao Y., Zhu H., Liu X. Effect of local alkaline microenvironment on the behaviors of bacteria and osteogenic cells. ACS Appl. Mater. Interfaces. 2018;10(49):42018–42029. doi: 10.1021/acsami.8b15724. [DOI] [PubMed] [Google Scholar]
- 169.Orrego S., Chen Z., Krekora U., Hou D., Jeon S.Y., Pittman M., Montoya C., Chen Y., Kang S.H. Bioinspired materials with self‐adaptable mechanical properties. Adv. Mater. 2020;32(21) doi: 10.1002/adma.201906970. [DOI] [PubMed] [Google Scholar]
- 170.Liu Z., Wan X., Wang Z.L., Li L. Electroactive biomaterials and systems for cell fate determination and tissue regeneration: design and applications. Adv. Mater. 2021;33(32) doi: 10.1002/adma.202007429. [DOI] [PubMed] [Google Scholar]
- 171.Wei H., Cui J., Lin K., Xie J., Wang X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Research. 2022;10(1):17. doi: 10.1038/s41413-021-00180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang J., Jia Q., Yue Z., Huo J., Chai J., Yu L., Nie R., Shao H., Zhao Y., Li P., Huang W. An electroluminodynamic flexible device for highly efficient eradication of drug-resistant bacteria. Adv. Mater. 2022;34(17) doi: 10.1002/adma.202200334. [DOI] [PubMed] [Google Scholar]
- 173.Fang J., Wan Y., Sun Y., Sun X., Qi M., Cheng S., Li C., Zhou Y., Xu L., Dong B., Wang L. Near-infrared-activated nanohybrid coating with black phosphorus/zinc oxide for efficient biofilm eradication against implant-associated infections. Chem. Eng. J. 2022;435 doi: 10.1016/j.cej.2022.134935. [DOI] [Google Scholar]
- 174.Xu Y., Liu X., Zheng Y., Li C., Kwok Yeung K.W., Cui Z., Liang Y., Li Z., Zhu S., Wu S. Ag3PO4 decorated black urchin-like defective TiO2 for rapid and long-term bacteria-killing under visible light. Bioact. Mater. 2021;6(6):1575–1587. doi: 10.1016/j.bioactmat.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tong L., Liao Q., Zhao Y., Huang H., Gao A., Zhang W., Gao X., Wei W., Guan M., Chu P.K. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials. 2019;193:1–11. doi: 10.1016/j.biomaterials.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 176.Wu Y., Liao Q., Wu L., Luo Y., Zhang W., Guan M., Pan H., Tong L., Chu P.K., Wang H. ZnL2-BPs integrated bone scaffold under sequential photothermal mediation: a win-win strategy delivering antibacterial therapy and fostering osteogenesis thereafter. ACS Nano. 2021;15(11):17854–17869. doi: 10.1021/acsnano.1c06062. [DOI] [PubMed] [Google Scholar]
- 177.Zhang D., Peng F., Tan J., Zhang Y., Wang F., Xie J., Xu R., Du H., Qian S., Qiao Y., Li M., Liu X. Self-assembled ferric oxyhydroxide nanosheet on PEO-coated magnesium alloy with photocatalytic/photothermal antibacterial and enhanced osteogenesis activities. Chem. Eng. J. 2022;437 doi: 10.1016/j.cej.2022.135257. [DOI] [Google Scholar]
- 178.Huang D., Wang J., Song C., Zhao Y. Ultrasound-responsive matters for biomedical applications. Innovation. 2023;4(3) doi: 10.1016/j.xinn.2023.100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Feng P., He R., Gu Y., Yang F., Pan H., Shuai C. Construction of antibacterial bone implants and their application in bone regeneration. Mater. Horiz. 2024;11(3):590–625. doi: 10.1039/D3MH01298K. [DOI] [PubMed] [Google Scholar]
- 180.Su K., Tan L., Liu X., Cui Z., Zheng Y., Li B., Han Y., Li Z., Zhu S., Liang Y., Feng X., Wang X., Wu S. Rapid photo-sonotherapy for clinical treatment of bacterial infected bone implants by creating oxygen deficiency using sulfur doping. ACS Nano. 2020;14(2):2077–2089. doi: 10.1021/acsnano.9b08686. [DOI] [PubMed] [Google Scholar]
- 181.Wu W., Mao D., Cai X., Duan Y., Hu F., Kong D., Liu B. ONOO– and ClO– responsive organic nanoparticles for specific in vivo image-guided photodynamic bacterial ablation. Chem. Mater. 2018;30(11):3867–3873. doi: 10.1021/acs.chemmater.8b01320. [DOI] [Google Scholar]
- 182.Carpenter A.W., Schoenfisch M.H. Nitric oxide release: part II. Therapeutic applications. Chem. Soc. Rev. 2012;41(10):3742–3752. doi: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nguyen T.K., Selvanayagam R., Ho K.K.K., Chen R., Kutty S.K., Rice S.A., Kumar N., Barraud N., Duong H.T.T., Boyer C. Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles. Chem. Sci. 2016;7(2):1016–1027. doi: 10.1039/c5sc02769a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li Y., Liu X., Li B., Zheng Y., Han Y., Chen D.F., Yeung K.W.K., Cui Z., Liang Y., Li Z., Zhu S., Wang X., Wu S. Near-infrared light triggered phototherapy and immunotherapy for elimination of methicillin-resistant Staphylococcus aureus biofilm infection on bone implant. ACS Nano. 2020;14(7):8157–8170. doi: 10.1021/acsnano.0c01486. [DOI] [PubMed] [Google Scholar]
- 185.Xu K., Yuan Z., Ding Y., He Y., Li K., Lin C., Tao B., Yang Y., Li X., Liu P., Cai K. Near-infrared light triggered multi-mode synergetic therapy for improving antibacterial and osteogenic activity of titanium implants. Appl. Mater. Today. 2021;24 doi: 10.1016/j.apmt.2021.101155. [DOI] [Google Scholar]
- 186.Wei T., Yu Q., Chen H. Responsive and synergistic antibacterial coatings: fighting against bacteria in a smart and effective way. Adv. Healthcare Mater. 2019;8(3) doi: 10.1002/adhm.201801381. [DOI] [PubMed] [Google Scholar]
- 187.Merkl P., Aschtgen M.S., Henriques-Normark B., Sotiriou G.A. Biofilm interfacial acidity evaluation by pH-Responsive luminescent nanoparticle films. Biosens. Bioelectron. 2021;171 doi: 10.1016/j.bios.2020.112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cao J., Zhao Y., Liu Y., Tian S., Zheng C., Liu C., Zhai Y., An Y., Busscher H.J., Shi L., Liu Y. Phosphorylcholine-based polymer encapsulated chitosan nanoparticles enhance the penetration of antimicrobials in a staphylococcal biofilm. ACS Macro Lett. 2019;8(6):651–657. doi: 10.1021/acsmacrolett.9b00142. [DOI] [PubMed] [Google Scholar]
- 189.Zhang F., Hu Q., Wei Y., Meng W., Wang R., Liu J., Nie Y., Luo R., Wang Y., Shen B. Surface modification of titanium implants by pH-Responsive coating designed for Self-Adaptive antibacterial and promoted osseointegration. Chem. Eng. J. 2022;435 doi: 10.1016/j.cej.2022.134802. [DOI] [Google Scholar]
- 190.Slavin Y.N., Asnis J., Hafeli U.O., Bach H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017;15(1):65. doi: 10.1186/s12951-017-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang L., Yang Q., Huo M., Lu D., Gao Y., Chen Y., Xu H. Engineering single-atomic iron-catalyst-integrated 3D-printed bioscaffolds for osteosarcoma destruction with antibacterial and bone defect regeneration bioactivity. Adv. Mater. 2021;33(31) doi: 10.1002/adma.202100150. [DOI] [PubMed] [Google Scholar]
- 192.Yu S., Li G., Zhao P., Cheng Q., He Q., Ma D., Xue W. NIR‐Laser‐Controlled hydrogen‐releasing PdH nanohydride for synergistic hydrogen‐photothermal antibacterial and wound‐healing therapies. Adv. Funct. Mater. 2019;29(50) doi: 10.1002/adfm.201905697. [DOI] [Google Scholar]
- 193.Hu J., Ding Y., Tao B., Yuan Z., Yang Y., Xu K., Li X., Liu P., Cai K. Surface modification of titanium substrate via combining photothermal therapy and quorum-sensing-inhibition strategy for improving osseointegration and treating biofilm-associated bacterial infection. Bioact. Mater. 2022;18:228–241. doi: 10.1016/j.bioactmat.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dedeogullari E.S., Caglar O., Danisman M., Tokgozoglu A.M., Kamaci S., Atilla B. Low dose vancomycin-loaded spacers for two-stage revision knee arthroplasty: high success, low toxicity. Knee. 2023;40:63–70. doi: 10.1016/j.knee.2022.11.008. [DOI] [PubMed] [Google Scholar]
- 195.Kumar M., Kumar R., Kumar S. Coatings on orthopedic implants to overcome present problems and challenges: a focused review. Mater. Today Proc. 2021;45:5269–5276. doi: 10.1016/j.matpr.2021.01.831. [DOI] [Google Scholar]
- 196.Cadosch D., Chan E., Gautschi O.P., Filgueira L. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening--current concepts. J. Biomed. Mater. Res. 2009;91(4):1252–1262. doi: 10.1002/jbm.a.32625. [DOI] [PubMed] [Google Scholar]
- 197.Bekmurzayeva A., Duncanson W.J., Azevedo H.S., Kanayeva D. Surface modification of stainless steel for biomedical applications: revisiting a century-old material. Mater. Sci. Eng. C. 2018;93:1073–1089. doi: 10.1016/j.msec.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 198.Asl S.M., Ganjali M., Karimi M. Surface modification of 316L stainless steel by laser-treated HA-PLA nanocomposite films toward enhanced biocompatibility and corrosion-resistance in vitro. Surf. Coating. Technol. 2019;363:236–243. doi: 10.1016/j.surfcoat.2019.02.052. [DOI] [Google Scholar]
- 199.Lamichhane Y., Singh G., Bhui A.S., Mukhiya P., Kumar P., Thapa B. Surface modification of 316L SS with HAp nano-particles using PMEDM for enhanced Biocompatibility. Mater. Today Proc. 2019;15:336–343. doi: 10.1016/j.matpr.2019.05.014. [DOI] [Google Scholar]
- 200.Mohammadzadeh Asl S., Ganjali M., Karimi M. Surface modification of 316L stainless steel by laser-treated HA-PLA nanocomposite films toward enhanced biocompatibility and corrosion-resistance in vitro. Surf. Coating. Technol. 2019;363:236–243. doi: 10.1016/j.surfcoat.2019.02.052. [DOI] [Google Scholar]
- 201.Sang W., Zhang Z., Dai Y., Chen X. Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chem. Soc. Rev. 2019;48(14):3771–3810. doi: 10.1039/c8cs00896e. [DOI] [PubMed] [Google Scholar]
- 202.Chen Z., Han S., Shi M., Liu G., Chen Z., Chang J., Wu C., Xiao Y. Immunomodulatory effects of mesoporous silica nanoparticles on osteogenesis: from nanoimmunotoxicity to nanoimmunotherapy. Appl. Mater. Today. 2018;10:184–193. doi: 10.1016/j.apmt.2017.12.003. [DOI] [Google Scholar]
- 203.Chen Z., Klein T., Murray R.Z., Crawford R., Chang J., Wu C., Xiao Y. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today. 2016;19(6):304–321. doi: 10.1016/j.mattod.2015.11.004. [DOI] [Google Scholar]
- 204.Niu Y., Li Q., Xie R., Liu S., Wang R., Xing P., Shi Y., Wang Y., Dong L., Wang C. Modulating the phenotype of host macrophages to enhance osteogenesis in MSC-laden hydrogels: design of a glucomannan coating material. Biomaterials. 2017;139:39–55. doi: 10.1016/j.biomaterials.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 205.Peña O.A., Martin P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024;25(8):599–616. doi: 10.1038/s41580-024-00715-1. [DOI] [PubMed] [Google Scholar]
- 206.Bi Z., Cai Y., Shi X., Chen J., Li D., Zhang P., Liu J. Macrophage-mediated immunomodulation in biomaterial-assisted bone repair: molecular insights and therapeutic prospects. Chem. Eng. J. 2024;488 doi: 10.1016/j.cej.2024.150631. [DOI] [Google Scholar]
- 207.Zhang B., Su Y., Zhou J., Zheng Y., Zhu D. Toward a better regeneration through implant-mediated immunomodulation: harnessing the immune responses. Adv. Sci. 2021;8(16) doi: 10.1002/advs.202100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li S., Yang H., Qu X., Qin Y., Liu A., Bao G., Huang H., Sun C., Dai J., Tan J., Shi J., Guan Y., Pan W., Gu X., Jia B., Wen P., Wang X., Zheng Y. Multiscale architecture design of 3D printed biodegradable Zn-based porous scaffolds for immunomodulatory osteogenesis. Nat. Commun. 2024;15(1):3131. doi: 10.1038/s41467-024-47189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sridharan R., Cameron A.R., Kelly D.J., Kearney C.J., O'Brien F.J. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater. Today. 2015;18(6):313–325. doi: 10.1016/j.mattod.2015.01.019. [DOI] [Google Scholar]
- 211.Lourenço A.H., Torres A.L., Vasconcelos D.P., Ribeiro-Machado C., Barbosa J.N., Barbosa M.A., Barrias C.C., Ribeiro C.C. Osteogenic, anti-osteoclastogenic and immunomodulatory properties of a strontium-releasing hybrid scaffold for bone repair. Mater. Sci. Eng. C. 2019;99:1289–1303. doi: 10.1016/j.msec.2019.02.053. [DOI] [PubMed] [Google Scholar]
- 212.Manilay J.O., Zouali M. Tight relationships between B lymphocytes and the skeletal system. Trends Mol. Med. 2014;20(7):405–412. doi: 10.1016/j.molmed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 213.Tsukasaki M., Takayanagi H. Osteoimmunology: evolving concepts in bone–immune interactions in health and disease. Nat. Rev. Immunol. 2019;19(10):626–642. doi: 10.1038/s41577-019-0178-8. [DOI] [PubMed] [Google Scholar]
- 214.Vishwakarma A., Bhise N.S., Evangelista M.B., Rouwkema J., Dokmeci M.R., Ghaemmaghami A.M., Vrana N.E., Khademhosseini A. Engineering immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol. 2016;34(6):470–482. doi: 10.1016/j.tibtech.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 215.Haumer A., Bourgine P.E., Occhetta P., Born G., Tasso R., Martin I. Delivery of cellular factors to regulate bone healing. Adv. Drug Deliv. Rev. 2018;129:285–294. doi: 10.1016/j.addr.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 216.Balmayor E.R. Targeted delivery as key for the success of small osteoinductive molecules. Adv. Drug Deliv. Rev. 2015;94:13–27. doi: 10.1016/j.addr.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 217.Xiang G., Liu K., Wang T., Hu X., Wang J., Gao Z., Lei W., Feng Y., Tao T.H. In situ regulation of macrophage polarization to enhance osseointegration under diabetic conditions using injectable silk/sitagliptin gel scaffolds. Adv. Sci. 2021;8(3) doi: 10.1002/advs.202002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhu F., Ji L., Dai K., Deng S., Wang J., Liu C. In situ licensing of mesenchymal stem cell immunomodulatory function via BMP-2 induced developmental process. Proc. Natl. Acad. Sci. U. S. A. 2024;121(48) doi: 10.1073/pnas.2410579121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 220.Sun X., Ma Z., Zhao X., Jin W., Zhang C., Ma J., Qiang L., Wang W., Deng Q., Yang H., Zhao J., Liang Q., Zhou X., Li T., Wang J. Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus. Bioact. Mater. 2021;6(3):757–769. doi: 10.1016/j.bioactmat.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cockerill I., Su Y., Lee J.H., Berman D., Young M.L., Zheng Y., Zhu D. Micro-/Nanotopography on bioresorbable zinc dictates cytocompatibility, bone cell differentiation, and macrophage polarization. Nano Lett. 2020;20(6):4594–4602. doi: 10.1021/acs.nanolett.0c01448. [DOI] [PubMed] [Google Scholar]
- 222.Gao A., Liao Q., Xie L., Wang G., Zhang W., Wu Y., Li P., Guan M., Pan H., Tong L. Tuning the surface immunomodulatory functions of polyetheretherketone for enhanced osseointegration. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119642. [DOI] [PubMed] [Google Scholar]
- 223.Yu D., Guo S., Yu M., Liu W., Li X., Chen D., Li B., Guo Z., Han Y. Immunomodulation and osseointegration activities of Na2TiO3 nanorods-arrayed coatings doped with different Sr content. Bioact. Mater. 2022;10:323–334. doi: 10.1016/j.bioactmat.2021.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Xu Y., Zhang L., Xu J., Li J., Wang H., He F. Strontium‐incorporated titanium implant surfaces treated by hydrothermal treatment enhance rapid osseointegration in diabetes: a preclinical vivo experimental study. Clin. Oral Implants Res. 2021;32(11):1366–1383. doi: 10.1111/clr.13837. [DOI] [PubMed] [Google Scholar]
- 225.Zhou Y., Wu C., Chang J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater. Today. 2019;24:41–56. doi: 10.1016/j.mattod.2018.07.016. [DOI] [Google Scholar]
- 226.Zhou P., Xia D., Ni Z., Ou T., Wang Y., Zhang H., Mao L., Lin K., Xu S., Liu J. Calcium silicate bioactive ceramics induce osteogenesis through oncostatin M. Bioact. Mater. 2021;6(3):810–822. doi: 10.1016/j.bioactmat.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wan Q.-Q., Sun J.-L., Ma Y.-X., Noble L.C., Dong Y., Feng Z.-H., Gu J.-T., Wang Y.-R., Wang W.-R., Bergeron B.E., Jiao K., Tay F.R., Niu L.-N. Immunomodulatory effects of tricalcium silicate-based cements on osteogenesis. Appl. Mater. Today. 2021;24 doi: 10.1016/j.apmt.2021.101145. [DOI] [Google Scholar]
- 228.Lee J., Byun H., Madhurakkat Perikamana S.K., Lee S., Shin H. Current advances in immunomodulatory biomaterials for bone regeneration. Adv. Healthcare Mater. 2019;8(4) doi: 10.1002/adhm.201801106. [DOI] [PubMed] [Google Scholar]
- 229.Yamada M., Kimura T., Nakamura N., Watanabe J., Kartikasari N., He X., Tiskratok W., Yoshioka H., Shinno H., Egusa H. Titanium Nanosurface with a biomimetic physical microenvironment to induce endogenous regeneration of the Periodontium. ACS Appl. Mater. Interfaces. 2022;14(24):27703–27719. doi: 10.1021/acsami.2c06679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jin S.-S., He D.-Q., Luo D., Wang Y., Yu M., Guan B., Fu Y., Li Z.-X., Zhang T., Zhou Y.-H. A biomimetic hierarchical nanointerface orchestrates macrophage polarization and mesenchymal stem cell recruitment to promote endogenous bone regeneration. ACS Nano. 2019;13(6):6581–6595. doi: 10.1021/acsnano.9b00489. [DOI] [PubMed] [Google Scholar]
- 231.Kong Y., Liu F., Ma B., Duan J., Yuan W., Sang Y., Han L., Wang S., Liu H. Wireless localized electrical stimulation generated by an ultrasound-driven piezoelectric Discharge regulates proinflammatory macrophage polarization. Adv. Sci. 2021;8(13) doi: 10.1002/advs.202100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dai X., Heng B.C., Bai Y., You F., Sun X., Li Y., Tang Z., Xu M., Zhang X., Deng X. Restoration of electrical microenvironment enhances bone regeneration under diabetic conditions by modulating macrophage polarization. Bioact. Mater. 2021;6(7):2029–2038. doi: 10.1016/j.bioactmat.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Reddy M.A., Chen Z., Park J.T., Wang M., Lanting L., Zhang Q., Bhatt K., Leung A., Wu X., Putta S., Sætrom P., Devaraj S., Natarajan R. Regulation of inflammatory phenotype in macrophages by a diabetes-induced long Noncoding RNA. Diabetes. 2014;63(12):4249–4261. doi: 10.2337/db14-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shuai C., Liu G., Yang Y., Qi F., Peng S., Yang W., He C., Wang G., Qian G. A strawberry-like Ag-decorated barium titanate enhances piezoelectric and antibacterial activities of polymer scaffold. Nano Energy. 2020;74 doi: 10.1016/j.nanoen.2020.104825. [DOI] [Google Scholar]
- 235.Weaver J.C., Astumian R.D. The response of living cells to very weak electric fields: the thermal noise limit. Science. 1990;247(4941):459–462. doi: 10.1126/science.2300806. [DOI] [PubMed] [Google Scholar]
- 236.Wu H., Dong H., Tang Z., Chen Y., Liu Y., Wang M., Wei X., Wang N., Bao S., Yu D. Electrical stimulation of piezoelectric BaTiO3 coated Ti6Al4V scaffolds promotes anti-inflammatory polarization of macrophages and bone repair via MAPK/JNK inhibition and OXPHOS activation. Biomaterials. 2023;293 doi: 10.1016/j.biomaterials.2022.121990. [DOI] [PubMed] [Google Scholar]
- 237.Tang B., Zhang B., Zhuang J., Wang Q., Dong L., Cheng K., Weng W. Surface potential-governed cellular osteogenic differentiation on ferroelectric polyvinylidene fluoride trifluoroethylene films. Acta Biomater. 2018;74:291–301. doi: 10.1016/j.actbio.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 238.Li M., Chu X., Wang D., Jian L., Liu L., Yao M., Zhang D., Zheng Y., Liu X., Zhang Y., Peng F. Tuning the surface potential to reprogram immune microenvironment for bone regeneration. Biomaterials. 2022;282 doi: 10.1016/j.biomaterials.2022.121408. [DOI] [PubMed] [Google Scholar]
- 239.Qin H., Cao H., Zhao Y., Jin G., Cheng M., Wang J., Jiang Y., An Z., Zhang X., Liu X. Antimicrobial and osteogenic properties of silver-ion-implanted stainless steel. ACS Appl. Mater. Interfaces. 2015;7(20):10785–10794. doi: 10.1021/acsami.5b01310. [DOI] [PubMed] [Google Scholar]
- 240.Chen L., Wang D., Liu X., Yan B., Zhang H., Zhang X., Qiao Y., Qiu J., Liu X. Micro-galvanic effects of silver-containing titanium implants regulate the immune responses via activating voltage-gated calcium channels in macrophages. Chem. Eng. J. 2022;428 doi: 10.1016/j.cej.2021.131068. [DOI] [Google Scholar]
- 241.Kopyl S., Surmenev R., Surmeneva M., Fetisov Y., Kholkin A. Magnetoelectric effect: principles and applications in biology and medicine- a review. Mater. Today Bio. 2021;12 doi: 10.1016/j.mtbio.2021.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Liu W., Zhang F., Yan Y., Zhang C., Zhao H., Heng B.C., Huang Y., Shen Y., Zhang J., Chen L., Wen X., Deng X. Remote tuning of Built‐in magnetoelectric microenvironment to promote bone regeneration by modulating cellular exposure to Arginylglycylaspartic acid peptide. Adv. Funct. Mater. 2020;31(6) doi: 10.1002/adfm.202006226. [DOI] [Google Scholar]
- 243.Xiao L., Ma Y., Crawford R., Mendhi J., Zhang Y., Lu H., Zhao Q., Cao J., Wu C., Wang X., Xiao Y. The interplay between hemostasis and immune response in biomaterial development for osteogenesis. Mater. Today. 2022;54:202–224. doi: 10.1016/j.mattod.2022.02.010. [DOI] [Google Scholar]
- 244.Fan Q., Ma Q., Bai J., Xu J., Fei Z., Dong Z., Maruyama A., Leong K.W., Liu Z., Wang C. An implantable blood clot-based immune niche for enhanced cancer vaccination. Sci. Adv. 2020;6(39) doi: 10.1126/sciadv.abb4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Fan Q., Bai J., Shan H., Fei Z., Chen H., Xu J., Ma Q., Zhou X., Wang C. Implantable blood clot loaded with BMP-2 for regulation of osteoimmunology and enhancement of bone repair. Bioact. Mater. 2021;6(11):4014–4026. doi: 10.1016/j.bioactmat.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Huang R., Wang X., Zhou Y., Xiao Y. RANKL-induced M1 macrophages are involved in bone formation. Bone Research. 2017;5(1) doi: 10.1038/boneres.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lu L.Y., Loi F., Nathan K., Lin T.h., Pajarinen J., Gibon E., Nabeshima A., Cordova L., Jämsen E., Yao Z. Pro‐inflammatory M1 macrophages promote osteogenesis by mesenchymal stem cells via the COX‐2‐prostaglandin E2 pathway. J. Orthop. Res. 2017;35(11):2378–2385. doi: 10.1002/jor.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Vallés G., Bensiamar F., Maestro-Paramio L., García-Rey E., Vilaboa N., Saldaña L. Influence of inflammatory conditions provided by macrophages on osteogenic ability of mesenchymal stem cells. Stem Cell Res. Ther. 2020;11(1):1–15. doi: 10.1186/s13287-020-1578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Xie L., Wang G., Wu Y., Liao Q., Mo S., Ren X., Tong L., Zhang W., Guan M., Pan H. Programmed surface on poly (aryl-ether-ether-ketone) initiating immune mediation and fulfilling bone regeneration sequentially. Innovation. 2021;2(3) doi: 10.1016/j.xinn.2021.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li B., Liu F., Ye J., Cai X., Qian R., Zhang K., Zheng Y., Wu S., Han Y. Regulation of macrophage polarization through periodic photo‐thermal treatment to facilitate osteogenesis. Small. 2022;18(38) doi: 10.1002/smll.202202691. [DOI] [PubMed] [Google Scholar]
- 251.Lee H., Thirunavukkarasu G.K., Kim S., Lee J.Y. Remote induction of in situ hydrogelation in a deep tissue, using an alternating magnetic field and superparamagnetic nanoparticles. Nano Res. 2018;11(11):5997–6009. doi: 10.1007/s12274-018-2114-9. [DOI] [Google Scholar]
- 252.Lin H., Gao S., Dai C., Chen Y., Shi J. A two-dimensional biodegradable Niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II Biowindows. J. Am. Chem. Soc. 2017;139(45):16235–16247. doi: 10.1021/jacs.7b07818. [DOI] [PubMed] [Google Scholar]
- 253.Cao Z., Wang D., Li Y., Xie W., Wang X., Tao L., Wei Y., Wang X., Zhao L. Effect of nanoheat stimulation mediated by magnetic nanocomposite hydrogel on the osteogenic differentiation of mesenchymal stem cells. Sci. China Life Sci. 2018;61(4):448–456. doi: 10.1007/s11427-017-9287-8. [DOI] [PubMed] [Google Scholar]
- 254.Liu X., Zheng J., Sun W., Zhao X., Li Y., Gong N., Wang Y., Ma X., Zhang T., Zhao L.-Y. Ferrimagnetic vortex nanoring-mediated mild magnetic hyperthermia imparts potent immunological effect for treating cancer metastasis. ACS Nano. 2019;13(8):8811–8825. doi: 10.1021/acsnano.9b01979. [DOI] [PubMed] [Google Scholar]
- 255.Huang D., Xu K., Huang X., Lin N., Ye Y., Lin S., Zhang J., Shao J., Chen S., Shi M., Zhou X., Lin P., Xue Y., Yu C., Yu X., Ye Z., Cheng K. Remotely temporal Scheduled macrophage phenotypic transition enables optimized immunomodulatory bone regeneration. Small. 2022;18(39) doi: 10.1002/smll.202203680. [DOI] [PubMed] [Google Scholar]
- 256.Herath T.D., Saigo L., Schaller B., Larbi A., Teoh S.H., Kirkpatrick C.J., Goh B.T. In vivo efficacy of neutrophil-mediated bone regeneration using a rabbit calvarial defect model. Int. J. Mol. Sci. 2021;22(23) doi: 10.3390/ijms222313016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhang L., Ke J., Wang Y., Yang S., Miron R.J., Zhang Y. A n in vitro investigation of the marked impact of dendritic cell interactions with bone grafts. J. Biomed. Mater. Res. 2017;105(6):1703–1711. doi: 10.1002/jbm.a.36048. [DOI] [PubMed] [Google Scholar]
- 258.Almeida C.R., Vasconcelos D.P., Gonçalves R.M., Barbosa M.A. Enhanced mesenchymal stromal cell recruitment via natural killer cells by incorporation of inflammatory signals in biomaterials. J. R. Soc. Interface. 2012;9(67):261–271. doi: 10.1098/rsif.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Cai B., Lin D., Li Y., Wang L., Xie J., Dai T., Liu F., Tang M., Tian L., Yuan Y., Kong L., Shen S.G.F. N2-Polarized neutrophils guide bone mesenchymal stem cell recruitment and initiate bone regeneration: a Missing Piece of the bone regeneration Puzzle. Adv. Sci. 2021;8(19) doi: 10.1002/advs.202100584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Khalmuratova R., Shin H.W., Kim D.W., Park J.W. Interleukin (IL)-13 and IL-17A contribute to neo-osteogenesis in chronic rhinosinusitis by inducing RUNX2. EBioMedicine. 2019;46:330–341. doi: 10.1016/j.ebiom.2019.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hotchkiss K.M., Clark N.M., Olivares-Navarrete R. Macrophage response to hydrophilic biomaterials regulates MSC recruitment and T-helper cell populations. Biomaterials. 2018;182:202–215. doi: 10.1016/j.biomaterials.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 262.Yu F., Lian R., Liu L., Liu T., Bi C., Hong K., Zhang S., Ren J., Wang H., Ouyang N., Du L.J., Liu Y., Zhou L., Liu Y., Fang B., Li Y., Duan S.Z., Xia L. Biomimetic hydroxyapatite nanorods promote bone regeneration via accelerating osteogenesis of BMSCs through T cell-derived IL-22. ACS Nano. 2022;16(1):755–770. doi: 10.1021/acsnano.1c08281. [DOI] [PubMed] [Google Scholar]
- 263.Iwasaki A., Medzhitov R. Regulation of adaptive immunity by the innate immune system. science. 2010;327(5963):291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhao Q., Shi M., Yin C., Zhao Z., Zhang J., Wang J., Shen K., Zhang L., Tang H., Xiao Y., Zhang Y. Dual-wavelength photosensitive nano-in-micro scaffold regulates innate and adaptive immune responses for osteogenesis. Nano-Micro Lett. 2020;13(1):28. doi: 10.1007/s40820-020-00540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Chung L., Maestas D.R., Jr., Housseau F., Elisseeff J.H. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2017;114:184–192. doi: 10.1016/j.addr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 266.Cekici A., Kantarci A., Hasturk H., Van Dyke T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology. 2014;64(1):57–80. doi: 10.1111/prd.12002. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Pirosa A., Gottardi R., Alexander P.G., Puppi D., Chiellini F., Tuan R.S. An in vitro chondro-osteo-vascular triphasic model of the osteochondral complex. Biomaterials. 2021;272 doi: 10.1016/j.biomaterials.2021.120773. [DOI] [PubMed] [Google Scholar]
- 268.Liu W.C., Chen S., Zheng L., Qin L. Angiogenesis Assays for the evaluation of angiogenic properties of orthopaedic biomaterials - a general review. Adv. Healthcare Mater. 2017;6(5) doi: 10.1002/adhm.201600434. [DOI] [PubMed] [Google Scholar]
- 269.Carano R.A.D., Filvaroff E.H. Angiogenesis and bone repair. Drug Discov. Today. 2003;8(21):980–989. doi: 10.1016/s1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- 270.Diao J., OuYang J., Deng T., Liu X., Feng Y., Zhao N., Mao C., Wang Y. 3D‐plotted beta‐tricalcium phosphate scaffolds with smaller pore sizes improve in vivo bone regeneration and biomechanical properties in a critical‐sized calvarial defect rat model. Adv. Healthcare Mater. 2018;7(17) doi: 10.1002/adhm.201800441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yang Y., Yao Z., Sun Y., Nie Y., Zhang Y., Li Z., Luo Z., Zhang W., Wang X., Du Y. 3D-printed manganese dioxide incorporated scaffold promotes osteogenic-angiogenic coupling for refractory bone defect by remodeling osteo-regenerative microenvironment. Bioact. Mater. 2025;44:354–370. doi: 10.1016/j.bioactmat.2024.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zberg B., Uggowitzer P.J., Loffler J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009;8(11):887–891. doi: 10.1038/nmat2542. [DOI] [PubMed] [Google Scholar]
- 273.Li F.Y., Chaigne-Delalande B., Kanellopoulou C., Davis J.C., Matthews H.F., Douek D.C., Cohen J.I., Uzel G., Su H.C., Lenardo M.J. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature. 2011;475(7357):471–476. doi: 10.1038/nature10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bose S., Fielding G., Tarafder S., Bandyopadhyay A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013;31(10):594–605. doi: 10.1016/j.tibtech.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jiang S., Wang X., Ma Y., Zhou Y., Liu L., Yu F., Fang B., Lin K., Xia L., Cai M. Synergistic effect of micro-nano-hybrid surfaces and Sr doping on the osteogenic and angiogenic capacity of hydroxyapatite bioceramics scaffolds. Int. J. Nanomed. 2022;17:783–797. doi: 10.2147/IJN.S345357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.de Baaij J.H., Hoenderop J.G., Bindels R.J. Magnesium in man: implications for health and disease. Physiol. Rev. 2015;95(1):1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 277.Lin S., Yang G., Jiang F., Zhou M., Yin S., Tang Y., Tang T., Zhang Z., Zhang W., Jiang X. A magnesium-enriched 3D culture system that mimics the bone development microenvironment for vascularized bone regeneration. Adv. Sci. 2019;6(12) doi: 10.1002/advs.201900209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gao P., Fan B., Yu X., Liu W., Wu J., Shi L., Yang D., Tan L., Wan P., Hao Y., Li S., Hou W., Yang K., Li X., Guo Z. Biofunctional magnesium coated Ti6Al4V scaffold enhances osteogenesis and angiogenesis in vitro and in vivo for orthopedic application. Bioact. Mater. 2020;5(3):680–693. doi: 10.1016/j.bioactmat.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Shypylenko A., Pshyk A., Grześkowiak B., Medjanik K., Peplinska B., Oyoshi K., Pogrebnjak A., Jurga S., Coy E. Effect of ion implantation on the physical and mechanical properties of Ti-Si-N multifunctional coatings for biomedical applications. Mater. Des. 2016;110:821–829. doi: 10.1016/j.matdes.2016.08.050. [DOI] [Google Scholar]
- 280.Yamaguchi S., Nath S., Matsushita T., Kokubo T. Controlled release of strontium ions from a bioactive Ti metal with a Ca-enriched surface layer. Acta Biomater. 2014;10(5):2282–2289. doi: 10.1016/j.actbio.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 281.Wan Z., Yuan Z., Li Y., Zhang Y., Wang Y., Yu Y., Mao J., Cai Q., Yang X. Hierarchical therapeutic ion‐based microspheres with precise ratio‐controlled delivery as Microscaffolds for in situ vascularized bone regeneration. Adv. Funct. Mater. 2022;32(23) doi: 10.1002/adfm.202113280. [DOI] [Google Scholar]
- 282.Zheng Y.F., Gu X.N., Witte F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014;77:1–34. doi: 10.1016/j.mser.2014.01.001. [DOI] [Google Scholar]
- 283.Shuai C., Li S., Peng S., Feng P., Lai Y., Gao C. Biodegradable metallic bone implants. Mater. Chem. Front. 2019;3(4):544–562. doi: 10.1039/C8QM00507A. [DOI] [Google Scholar]
- 284.Yin J., Pan S., Guo X., Gao Y., Zhu D., Yang Q., Gao J., Zhang C., Chen Y. Nb2C MXene-functionalized scaffolds enables osteosarcoma Phototherapy and angiogenesis/osteogenesis of bone defects. Nano-Micro Lett. 2021;13(1):30. doi: 10.1007/s40820-020-00547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Han H.S., Jun I., Seok H.K., Lee K.S., Lee K., Witte F., Mantovani D., Kim Y.C., Glyn-Jones S., Edwards J.R. Biodegradable magnesium alloys promote angio-osteogenesis to enhance bone repair. Adv. Sci. 2020;7(15) doi: 10.1002/advs.202000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Liu Y., Li H., Xu J., TerBush J., Li W., Setty M., Guan S., Nguyen T.D., Qin L., Zheng Y. Biodegradable metal-derived magnesium and sodium enhances bone regeneration by angiogenesis aided osteogenesis and regulated biological apatite formation. Chem. Eng. J. 2021;410 doi: 10.1016/j.cej.2020.127616. [DOI] [Google Scholar]
- 287.Vo T.N., Kasper F.K., Mikos A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012;64(12):1292–1309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Goodman S.B., Yao Z., Keeney M., Yang F. The future of biologic coatings for orthopaedic implants. Biomaterials. 2013;34(13):3174–3183. doi: 10.1016/j.biomaterials.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Bose S., Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 2012;8(4):1401–1421. doi: 10.1016/j.actbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Rajendran A.K., Anthraper M.S.J., Hwang N.S., Rangasamy J. Osteogenesis and angiogenesis promoting bioactive ceramics. Mater. Sci. Eng. R Rep. 2024;159 doi: 10.1016/j.mser.2024.100801. [DOI] [Google Scholar]
- 291.Peng Y., Wu S., Li Y., Crane J.L. Type H blood vessels in bone modeling and remodeling. Theranostics. 2020;10(1):426–436. doi: 10.7150/thno.34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zhang J., Pan J., Jing W. Motivating role of type H vessels in bone regeneration. Cell Prolif. 2020;53(9) doi: 10.1111/cpr.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sun Y., Liu X., Zeng X., Wang L., Jin Z., Yeung K.W.K., Liu X., Ouyang L., Liao Y. Simvastatin-loaded sulfonated PEEK enhances angiogenesis and osteogenesis via miR-29cb2-mediated HIF-3α downregulation. Chem. Eng. J. 2022;448 doi: 10.1016/j.cej.2022.137738. [DOI] [Google Scholar]
- 295.Chen W., Xie G., Lu Y., Wang J., Feng B., Wang Q., Xu K., Bao J. An improved osseointegration of metal implants by pitavastatin loaded multilayer films with osteogenic and angiogenic properties. Biomaterials. 2022;280 doi: 10.1016/j.biomaterials.2021.121260. [DOI] [PubMed] [Google Scholar]
- 296.Furuta T., Miyaki S., Ishitobi H., Ogura T., Kato Y., Kamei N., Miyado K., Higashi Y., Ochi M. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med. 2016;5(12):1620–1630. doi: 10.5966/sctm.2015-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Nakamura Y., Miyaki S., Ishitobi H., Matsuyama S., Nakasa T., Kamei N., Akimoto T., Higashi Y., Ochi M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2015;589(11):1257–1265. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 298.Wu D., Chang X., Tian J., Kang L., Wu Y., Liu J., Wu X., Huang Y., Gao B., Wang H., Qiu G., Wu Z. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J. Nanobiotechnol. 2021;19(1):209. doi: 10.1186/s12951-021-00958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Chen L., Xiong Y., Hu Y., Yu C., Panayi A.C., Zhou W., Cao F., Sun Y., Liu M., Liu G., Xue H., Hu L., Mi B., Liu G. Regulatory T cell-exosomal miR-142-3p promotes angiogenesis and osteogenesis via TGFBR1/SMAD2 inhibition to accelerate fracture repair. Chem. Eng. J. 2022;427 doi: 10.1016/j.cej.2021.131419. [DOI] [Google Scholar]
- 300.Zhang Y., Xie Y., Hao Z., Zhou P., Wang P., Fang S., Li L., Xu S., Xia Y. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl. Mater. Interfaces. 2021;13(16):18472–18487. doi: 10.1021/acsami.0c22671. [DOI] [PubMed] [Google Scholar]
- 301.Kim H.Y., Kwon S., Um W., Shin S., Kim C.H., Park J.H., Kim B.S. Functional extracellular vesicles for regenerative medicine. Small. 2022;18(36) doi: 10.1002/smll.202106569. [DOI] [PubMed] [Google Scholar]
- 302.Liu H., Li M., Zhang T., Liu X., Zhang H., Geng Z., Su J. Engineered bacterial extracellular vesicles for osteoporosis therapy. Chem. Eng. J. 2022;450 doi: 10.1016/j.cej.2022.138309. [DOI] [Google Scholar]
- 303.Tao S.C., Guo S.C., Zhang C.Q. Modularized extracellular vesicles: the dawn of prospective personalized and precision medicine. Adv. Sci. 2018;5(2) doi: 10.1002/advs.201700449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Tao S.C., Li X.R., Wei W.J., Wei Z.Y., Zhang C.R., Wang F., Dawes H., Guo S.C. Polymeric coating on beta-TCP scaffolds provides immobilization of small extracellular vesicles with surface-functionalization and ZEB1-Loading for bone defect repair in diabetes mellitus. Biomaterials. 2022;283 doi: 10.1016/j.biomaterials.2022.121465. [DOI] [PubMed] [Google Scholar]
- 305.Wang Y., Shim M.S., Levinson N.S., Sung H.W., Xia Y. Stimuli‐responsive materials for controlled release of theranostic agents. Adv. Funct. Mater. 2014;24(27):4206–4220. doi: 10.1002/adfm.201400279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Norgaard R., Kassem M., Rattan S.I. Heat shock-induced enhancement of osteoblastic differentiation of hTERT-immortalized mesenchymal stem cells. Ann. N. Y. Acad. Sci. 2006;1067:443–447. doi: 10.1196/annals.1354.063. [DOI] [PubMed] [Google Scholar]
- 307.Sayed S., Faruq O., Hossain M., Im S.B., Kim Y.S., Lee B.T. Thermal cycling effect on osteogenic differentiation of MC3T3-E1 cells loaded on 3D-porous Biphasic Calcium Phosphate (BCP) scaffolds for early osteogenesis. Mater. Sci. Eng. C. 2019;105 doi: 10.1016/j.msec.2019.110027. [DOI] [PubMed] [Google Scholar]
- 308.Cai Y., Wei Z., Song C., Tang C., Han W., Dong X. Optical nano-agents in the second near-infrared window for biomedical applications. Chem. Soc. Rev. 2019;48(1):22–37. doi: 10.1039/c8cs00494c. [DOI] [PubMed] [Google Scholar]
- 309.Miao Q., Pu K. Organic semiconducting agents for deep‐tissue molecular imaging: second near‐infrared fluorescence, self‐luminescence, and photoacoustics. Adv. Mater. 2018;30(49) doi: 10.1002/adma.201801778. [DOI] [PubMed] [Google Scholar]
- 310.Yang C., Ma H., Wang Z., Younis M.R., Liu C., Wu C., Luo Y., Huang P. 3D printed wesselsite nanosheets functionalized scaffold facilitates NIR-II photothermal therapy and vascularized bone regeneration. Adv. Sci. 2021;8(20) doi: 10.1002/advs.202100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wang L., Hu P., Jiang H., Zhao J., Tang J., Jiang D., Wang J., Shi J., Jia W. Mild hyperthermia-mediated osteogenesis and angiogenesis play a critical role in magnetothermal composite-induced bone regeneration. Nano Today. 2022;43 doi: 10.1016/j.nantod.2022.101401. [DOI] [Google Scholar]
- 312.Ha Y., Ma X., Li S., Li T., Li Z., Qian Y., Shafiq M., Wang J., Zhou X., He C. Bone microenvironment‐mimetic scaffolds with hierarchical microstructure for enhanced vascularization and bone regeneration. Adv. Funct. Mater. 2022;32(20) doi: 10.1002/adfm.202200011. [DOI] [Google Scholar]
- 313.Lukashev M.E., Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8(11):437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- 314.Studart A.R. Biological and bioinspired composites with spatially tunable heterogeneous architectures. Adv. Funct. Mater. 2013;23(36):4423–4436. doi: 10.1002/adfm.201300340. [DOI] [Google Scholar]
- 315.Ma L., Wang X., Zhou Y., Ji X., Cheng S., Bian D., Fan L., Zhou L., Ning C., Zhang Y. Biomimetic Ti-6Al-4V alloy/gelatin methacrylate hybrid scaffold with enhanced osteogenic and angiogenic capabilities for large bone defect restoration. Bioact. Mater. 2021;6(10):3437–3448. doi: 10.1016/j.bioactmat.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.He Y., Wang W., Lin S., Yang Y., Song L., Jing Y., Chen L., He Z., Li W., Xiong A., Yeung K.W.K., Zhao Q., Jiang Y., Li Z., Pei G., Zhang Z.Y. Fabrication of a bio-instructive scaffold conferred with a favorable microenvironment allowing for superior implant osseointegration and accelerated in situ vascularized bone regeneration via type H vessel formation. Bioact. Mater. 2022;9:491–507. doi: 10.1016/j.bioactmat.2021.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Quinlan E., Partap S., Azevedo M.M., Jell G., Stevens M.M., O'Brien F.J. Hypoxia-mimicking bioactive glass/collagen glycosaminoglycan composite scaffolds to enhance angiogenesis and bone repair. Biomaterials. 2015;52:358–366. doi: 10.1016/j.biomaterials.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 318.Carmeliet P., Dor Y., Herbert J.-M., Fukumura D., Brusselmans K., Dewerchin M., Neeman M., Bono F., Abramovitch R., Maxwell P. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394(6692):485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 319.Liang J.-P., Accolla R.P., Soundirarajan M., Emerson A., Coronel M.M., Stabler C.L. Engineering a macroporous oxygen-generating scaffold for enhancing islet cell transplantation within an extrahepatic site. Acta Biomater. 2021;130:268–280. doi: 10.1016/j.actbio.2021.05.028. [DOI] [PubMed] [Google Scholar]
- 320.Parmar K., Mauch P., Vergilio J.-A., Sackstein R., Down J.D. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl. Acad. Sci. 2007;104(13):5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Farzin A., Hassan S., Moreira Teixeira L.S., Gurian M., Crispim J.F., Manhas V., Carlier A., Bae H., Geris L., Noshadi I. Self‐oxygenation of tissues orchestrates Full‐Thickness vascularization of living implants. Adv. Funct. Mater. 2021;31(42) doi: 10.1002/adfm.202100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Yang C., Zheng Z., Younis M.R., Dong C., Chen Y., Lei S., Zhang D.Y., Wu J., Wu X., Lin J., Wang X., Huang P. 3D printed enzyme‐functionalized scaffold facilitates diabetic bone regeneration. Adv. Funct. Mater. 2021;31(20) doi: 10.1002/adfm.202101372. [DOI] [Google Scholar]
- 323.Stavropoulos A., Bertl K., Spineli L.M., Sculean A., Cortellini P., Tonetti M. Medium- and long-term clinical benefits of periodontal regenerative/reconstructive procedures in intrabony defects: systematic review and network meta-analysis of randomized controlled clinical studies. J. Clin. Periodontol. 2021;48(3):410–430. doi: 10.1111/jcpe.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Dimitriou R., Jones E., McGonagle D., Giannoudis P.V. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:1–10. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ekegren C.L., Edwards E.R., de Steiger R., Gabbe B.J. Incidence, Costs and Predictors of non-union, delayed union and Mal-union following long bone fracture. Int. J. Environ. Res. Publ. Health. 2018;15(12):2845. doi: 10.3390/ijerph15122845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 327.Pajarinen J., Lin T., Gibon E., Kohno Y., Maruyama M., Nathan K., Lu L., Yao Z., Goodman S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80–89. doi: 10.1016/j.biomaterials.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wu Y., Wang H., Chu P.K. Enhancing macrophages to combat intracellular bacteria. The Innovation Life. 2023;1(2):100027–100028. doi: 10.59717/j.xinn-life.2023.100027. [DOI] [Google Scholar]
- 329.Amin Yavari S., Castenmiller S.M., van Strijp J.A.G., Croes M. Combating implant infections: Shifting focus from bacteria to host. Adv. Mater. 2020;32(43) doi: 10.1002/adma.202002962. [DOI] [PubMed] [Google Scholar]
- 330.Zhang S., Yang H., Wang M., Mantovani D., Yang K., Witte F., Tan L., Yue B., Qu X. Immunomodulatory biomaterials against bacterial infections: progress, challenges, and future perspectives. Innovation. 2023;4(6) doi: 10.1016/j.xinn.2023.100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Fournier B., Philpott D.J. Recognition of Staphylococcus aureus by the innate immune system. Clin. Microbiol. Rev. 2005;18(3):521–540. doi: 10.1128/CMR.18.3.521-540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhang S., Chai Q., Man Z., Tang C., Li Z., Zhang J., Xu H., Xu X., Chen C., Liu Y., Guo F., Abdalla M., Yu G., Zhao K., Shi B., Li W., Jiang X. Bioinspired nano-painting on orthopedic implants orchestrates periprosthetic anti-infection and osseointegration in a rat model of arthroplasty. Chem. Eng. J. 2022;435 doi: 10.1016/j.cej.2022.134848. [DOI] [Google Scholar]
- 333.Tao B., Lin C., He Y., Yuan Z., Chen M., Xu K., Li K., Guo A., Cai K., Chen L. Osteoimmunomodulation mediating improved osteointegration by OGP-loaded cobalt-metal organic framework on titanium implants with antibacterial property. Chem. Eng. J. 2021;423 doi: 10.1016/j.cej.2021.130176. [DOI] [Google Scholar]
- 334.June C.H., Sadelain M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018;379(1):64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wu L., Wei Q., Brzostek J., Gascoigne N.R. Signaling from T cell receptors (TCRs) and chimeric antigen receptors (CARs) on T cells. Cell. Mol. Immunol. 2020;17(6):600–612. doi: 10.1038/s41423-020-0470-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Watanabe K., Terakura S., Uchiyama S., Martens A.C., van Meerten T., Kiyoi H., Nishida T., Naoe T., Murata M. Excessively high-affinity single-chain fragment variable region in a chimeric antigen receptor can counteract T-cell proliferation. Blood. 2014;124(21):4799. doi: 10.1182/blood.V124.21.4799.4799. [DOI] [Google Scholar]
- 337.Li Z., Zhang S., Fu Z., Liu Y., Man Z., Shi C., Tang C., Chen C., Chai Q., Yang Z. Surficial nano-deposition locoregionally yielding bactericidal super CAR-macrophages expedites periprosthetic osseointegration. Sci. Adv. 2023;9(22) doi: 10.1126/sciadv.adg3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Chen J., Zhu Y., Xiong M., Hu G., Zhan J., Li T., Wang L., Wang Y. Antimicrobial titanium surface via Click-immobilization of peptide and its in vitro/vivo activity. ACS Biomater. Sci. Eng. 2019;5(2):1034–1044. doi: 10.1021/acsbiomaterials.8b01046. [DOI] [PubMed] [Google Scholar]
- 339.Moussavian M.R., Laschke M.W., Schlachtenberger G., von Heesen M., Wagner M., Glanemann M., Menger M.D. Perigraft vascularization and incorporation of implanted Dacron prostheses are affected by rifampicin coating. J. Vasc. Surg. 2016;64(6):1815–1824. doi: 10.1016/j.jvs.2015.07.104. [DOI] [PubMed] [Google Scholar]
- 340.Kazemzadeh-Narbat M., Kindrachuk J., Duan K., Jenssen H., Hancock R.E., Wang R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials. 2010;31(36):9519–9526. doi: 10.1016/j.biomaterials.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 341.Kotsakis G.A., Lan C., Barbosa J., Lill K., Chen R., Rudney J., Aparicio C. Antimicrobial agents used in the treatment of peri‐implantitis alter the physicochemistry and cytocompatibility of titanium surfaces. J. Periodontol. 2016;87(7):809–819. doi: 10.1902/jop.2016.150684. [DOI] [PubMed] [Google Scholar]
- 342.Seo J.J., Mandakhbayar N., Kang M.S., Yoon J.Y., Lee N.H., Ahn J., Lee H.H., Lee J.H., Kim H.W. Antibacterial, proangiogenic, and osteopromotive nanoglass paste coordinates regenerative process following bacterial infection in hard tissue. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120593. [DOI] [PubMed] [Google Scholar]
- 343.Ryan E.J., Ryan A.J., González-Vázquez A., Philippart A., Ciraldo F.E., Hobbs C., Nicolosi V., Boccaccini A.R., Kearney C.J., O'Brien F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials. 2019;197:405–416. doi: 10.1016/j.biomaterials.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 344.Zhang Z., Wang Y., Teng W., Zhou X., Ye Y., Zhou H., Sun H., Wang F., Liu A., Lin P., Cui W., Yu X., Wu Y., Ye Z. An orthobiologics-free strategy for synergistic photocatalytic antibacterial and osseointegration. Biomaterials. 2021;274 doi: 10.1016/j.biomaterials.2021.120853. [DOI] [PubMed] [Google Scholar]
- 345.Sadowska J.M., Power R.N., Genoud K.J., Matheson A., González‐Vázquez A., Costard L., Eichholz K., Pitacco P., Hallegouet T., Chen G., Curtin C.M., Murphy C.M., Cavanagh B., Zhang H., Kelly D.J., Boccaccini A.R., O'Brien F.J. A multifunctional scaffold for bone infection treatment by delivery of microRNA therapeutics combined with antimicrobial nanoparticles. Adv. Mater. 2023;36(6) doi: 10.1002/adma.202307639. [DOI] [PubMed] [Google Scholar]
- 346.Liu W., Liu C., Liu C., Li Y., Pan L., Wang J., Jian X. Surface chemical modification of poly (phthalazinone ether nitrile ketone) through rhBMP-2 and antimicrobial peptide conjugation for enhanced osteogenic and antibacterial activities in vitro and in vivo. Chem. Eng. J. 2021;424 doi: 10.1016/j.cej.2021.130321. [DOI] [Google Scholar]
- 347.Kazemzadeh-Narbat M., Cheng H., Chabok R., Alvarez M.M., De La Fuente-Nunez C., Phillips K.S., Khademhosseini A. Strategies for antimicrobial peptide coatings on medical devices: a review and regulatory science perspective. Crit. Rev. Biotechnol. 2021;41(1):94–120. doi: 10.1080/07388551.2020.1828810. [DOI] [PubMed] [Google Scholar]
- 348.Chen J., Hu G., Li T., Chen Y., Gao M., Li Q., Hao L., Jia Y., Wang L., Wang Y. Fusion peptide engineered "statically-versatile" titanium implant simultaneously enhancing anti-infection, vascularization and osseointegration. Biomaterials. 2021;264 doi: 10.1016/j.biomaterials.2020.120446. [DOI] [PubMed] [Google Scholar]
- 349.Santulli G., Ciccarelli M., Palumbo G., Campanile A., Galasso G., Ziaco B., Altobelli G.G., Cimini V., Piscione F., D'Andrea L.D. In vivo properties of the proangiogenic peptide QK. J. Transl. Med. 2009;7(1):1–10. doi: 10.1186/1479-5876-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Cursiefen C., Chen L., Borges L.P., Jackson D., Cao J., Radziejewski C., D'Amore P.A., Dana M.R., Wiegand S.J., Streilein J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004;113(7):1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Bai L., Chen P., Zhao Y., Hang R., Yao X., Tang B., Liu C., Xiao Y., Hang R. A micro/nano-biomimetic coating on titanium orchestrates osteo/angio-genesis and osteoimmunomodulation for advanced osseointegration. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121162. [DOI] [PubMed] [Google Scholar]
- 352.Elrayah A., Zhi W., Feng S., Al-Ezzi S., Lei H., Weng J. Preparation of micro/nano-structure copper-substituted hydroxyapatite scaffolds with improved angiogenesis capacity for bone regeneration. Materials. 2018;11(9):1516. doi: 10.3390/ma11091516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Weng L., Boda S.K., Wang H., Teusink M.J., Shuler F.D., Xie J. Novel 3D hybrid nanofiber Aerogels coupled with BMP‐2 peptides for cranial bone regeneration. Adv. Healthcare Mater. 2018;7(10) doi: 10.1002/adhm.201701415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Zhang W., Chang Q., Xu L., Li G., Yang G., Ding X., Wang X., Cui D., Jiang X. Graphene oxide‐copper Nanocomposite‐coated porous CaP scaffold for vascularized bone regeneration via activation of Hif‐1α. Adv. Healthcare Mater. 2016;5(11):1299–1309. doi: 10.1002/adhm.201500824. [DOI] [PubMed] [Google Scholar]
- 355.Yan F., Yu M., He Y., Wang F., Yang F., Zhao X., Zheng Y., Liu Y., Xia D., Liu Y. Hierarchical mineralized collagen coated Zn membrane to tailor cell microenvironment for guided bone regeneration. Adv. Funct. Mater. 2025 doi: 10.1002/adfm.202412695. [DOI] [Google Scholar]
- 356.Zhou M., Wu X., Luo J., Yang G., Lu Y., Lin S., Jiang F., Zhang W., Jiang X. Copper peptide-incorporated 3D-printed silk-based scaffolds promote vascularized bone regeneration. Chem. Eng. J. 2021;422 doi: 10.1016/j.cej.2021.130147. [DOI] [Google Scholar]
- 357.Li X., Wei L., Li J., Shao J., Yi B., Zhang C., Liu H., Ma B., Ge S. Multifunctional SDF-1-loaded hydroxyapatite/polylactic acid membranes promote cell recruitment, immunomodulation, angiogenesis, and osteogenesis for biomimetic bone regeneration. Appl. Mater. Today. 2021;22 doi: 10.1016/j.apmt.2021.100942. [DOI] [Google Scholar]
- 358.Xiao S., Wei J., Jin S., Xia X., Yuan L., Zou Q., Zuo Y., Li J., Li Y. A multifunctional coating strategy for promotion of immunomodulatory and osteo/angio‐Genic activity. Adv. Funct. Mater. 2022;33(4) doi: 10.1002/adfm.202208968. [DOI] [Google Scholar]
- 359.Song B., Zhang C., Hu W., Guo C., Xia Z., Hu W., Qin M., Jiang W., Lv J., Xu D. Nano-designed carbon monoxide donor SMA/CORM2 exhibits protective effect against acetaminophen induced liver injury through macrophage reprograming and promoting liver regeneration. J. Contr. Release. 2021;331:350–363. doi: 10.1016/j.jconrel.2021.01.025. [DOI] [PubMed] [Google Scholar]
- 360.Otterbein L.E., Bach F.H., Alam J., Soares M., Tao Lu H., Wysk M., Davis R.J., Flavell R.A., Choi A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6(4):422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 361.Zhang J., Tong D., Song H., Ruan R., Sun Y., Lin Y., Wang J., Hou L., Dai J., Ding J., Yang H. Osteoimmunity-regulating biomimetically hierarchical scaffold for augmented bone regeneration. Adv. Mater. 2022;34(36) doi: 10.1002/adma.202202044. [DOI] [PubMed] [Google Scholar]
- 362.Zhao Q., Shi M., Yin C., Zhao Z., Zhang J., Wang J., Shen K., Zhang L., Tang H., Xiao Y. Dual-wavelength photosensitive nano-in-micro scaffold regulates innate and adaptive immune responses for osteogenesis. Nano-Micro Lett. 2021;13(1):1–20. doi: 10.1007/s40820-020-00540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Qiu P., Li M., Chen K., Fang B., Chen P., Tang Z., Lin X., Fan S. Periosteal matrix-derived hydrogel promotes bone repair through an early immune regulation coupled with enhanced angio-and osteogenesis. Biomaterials. 2020;227 doi: 10.1016/j.biomaterials.2019.119552. [DOI] [PubMed] [Google Scholar]
- 364.Wu Y., Liao Q., Wu L., Luo Y., Zhang W., Guan M., Pan H., Tong L., Chu P.K., Wang H. ZnL2-BPs integrated bone scaffold under sequential photothermal mediation: a win–win strategy delivering antibacterial therapy and fostering osteogenesis thereafter. ACS Nano. 2021;15(11):17854–17869. doi: 10.1021/acsnano.1c06062. [DOI] [PubMed] [Google Scholar]
- 365.Hankenson K.D., Dishowitz M., Gray C., Schenker M. Angiogenesis in bone regeneration. Injury. 2011;42(6):556–561. doi: 10.1016/j.injury.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Lyu Z., Zhao Y., Huo S., Wang F., Meng X., Yuan Z., Long T., Wang Y. Mussel-inspired dopamine-CuII coated polyetheretherketone surface with direct and immunomodulatory effect to facilitate osteogenesis, angiogenesis, and antibacterial ability. Mater. Des. 2022;222 doi: 10.1016/j.matdes.2022.111069. [DOI] [Google Scholar]
- 367.Huang R., Wang X., Zhou Y., Xiao Y. RANKL-induced M1 macrophages are involved in bone formation. Bone Research. 2017;5 doi: 10.1038/boneres.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Vallet‐Regí M., Lozano D., González B., Izquierdo‐Barba I. Biomaterials against bone infection. Adv. Healthcare Mater. 2020;9(13) doi: 10.1002/adhm.202000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Kalelkar P.P., Riddick M., García A.J. Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nat. Rev. Mater. 2022;7(1):39–54. doi: 10.1038/s41578-021-00362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Jian G., Li D., Ying Q., Chen X., Zhai Q., Wang S., Mei L., Cannon R.D., Ji P., Wang H. Dual photo-enhanced interpenetrating network hydrogel with biophysical and biochemical signals for infected bone defect healing. Adv. Healthcare Mater. 2023;12(25) doi: 10.21203/rs.3.rs-2534216/v1. [DOI] [PubMed] [Google Scholar]
- 371.Zhang Z., Wang F., Huang X., Sun H., Xu J., Qu H., Yan X., Shi W., Teng W., Jin X., Shao Z., Zhang Y., Zhao S., Wu Y., Ye Z., Yu X. Engineered sensory nerve guides self-adaptive bone healing via NGF-TrkA signaling pathway. Adv. Sci. 2023;10(10) doi: 10.1002/advs.202206155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Zhang Z., Hao Z., Xian C., Fang Y., Cheng B., Wu J., Xia J. Neuro-bone tissue engineering: multiple potential translational strategies between nerve and bone. Acta Biomater. 2022;153:1–12. doi: 10.1016/j.actbio.2022.09.023. [DOI] [PubMed] [Google Scholar]
- 373.Tao R., Mi B., Hu Y., Lin S., Xiong Y., Lu X., Panayi A.C., Li G., Liu G. Hallmarks of peripheral nerve function in bone regeneration. Bone Research. 2023;11(1):6. doi: 10.1038/s41413-022-00240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Wan Q.Q., Qin W.P., Ma Y.X., Shen M.J., Li J., Zhang Z.B., Chen J.H., Tay F.R., Niu L.N., Jiao K. Crosstalk between bone and nerves within bone. Adv. Sci. 2021;8(7) doi: 10.1002/advs.202003390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Liu W., Chen W., Xie M., Chen C., Shao Z., Zhang Y., Zhao H., Song Q., Hu H., Xing X. Traumatic brain injury stimulates sympathetic tone-mediated bone marrow myelopoiesis to favor fracture healing. Signal Transduct. Targeted Ther. 2023;8(1):260. doi: 10.1038/s41392-023-01457-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Fukuda T., Takeda S., Xu R., Ochi H., Sunamura S., Sato T., Shibata S., Yoshida Y., Gu Z., Kimura A. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497(7450):490–493. doi: 10.1038/nature12115. [DOI] [PubMed] [Google Scholar]
- 377.Marrella A., Lee T.Y., Lee D.H., Karuthedom S., Syla D., Chawla A., Khademhosseini A., Jang H.L. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today. 2018;21(4):362–376. doi: 10.1016/j.mattod.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Tong L., Wijnen A., Wang H., Chen D. Advancing bone biology: the mutual promotion of biology and pioneering technologies. The Innovation Life. 2024;2(3) doi: 10.59717/j.xinn-life.2024.100078. [DOI] [Google Scholar]
- 379.Takeda S., Elefteriou F., Levasseur R., Liu X., Zhao L., Parker K.L., Armstrong D., Ducy P., Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 380.Elefteriou F. Impact of the autonomic nervous system on the skeleton. Physiol. Rev. 2018;98(3):1083–1112. doi: 10.1152/physrev.00014.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Kim J.H., Jung Y., Kim B.-S., Kim S.H. Stem cell recruitment and angiogenesis of neuropeptide substance P coupled with self-assembling peptide nanofiber in a mouse hind limb ischemia model. Biomaterials. 2013;34(6):1657–1668. doi: 10.1016/j.biomaterials.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 382.Zhang Y., Xu J., Ruan Y.C., Yu M.K., O'Laughlin M., Wise H., Chen D., Tian L., Shi D., Wang J. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016;22(10):1160–1169. doi: 10.1038/nm.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Zhang Y., Chen C.Y., Liu Y.W., Rao S.S., Tan Y.J., Qian Y.X., Xia K., Huang J., Liu X.X., Hong C.G. Neuronal induction of bone‐fat imbalance through osteocyte neuropeptide Y. Adv. Sci. 2021;8(24) doi: 10.1002/advs.202100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Wang X.-D., Li S.-Y., Zhang S.-J., Gupta A., Zhang C.-P., Wang L. The neural system regulates bone homeostasis via mesenchymal stem cells: a translational approach. Theranostics. 2020;10(11):4839. doi: 10.7150/thno.43771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Xu Y., Xu C., He L., Zhou J., Chen T., Ouyang L., Guo X., Qu Y., Luo Z., Duan D. Stratified-structural hydrogel incorporated with magnesium-ion-modified black phosphorus nanosheets for promoting neuro-vascularized bone regeneration. Bioact. Mater. 2022;16:271–284. doi: 10.1016/j.bioactmat.2022.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Guo S., He C. Bioprinted scaffold Remodels the neuromodulatory microenvironment for enhancing bone regeneration. Adv. Funct. Mater. 2023;33(40) doi: 10.1002/adfm.202304172. [DOI] [Google Scholar]
- 387.Wang T., Li W., Zhang Y., Xu X., Qiang L., Miao W., Yue X., Jiao X., Zhou X., Ma Z., Li S., Ding M., Zhu J., Yang C., Wang H., Li T., Sun X., Wang J. Bioprinted constructs that simulate nerve-bone crosstalk to improve microenvironment for bone repair. Bioact. Mater. 2023;27:377–393. doi: 10.1016/j.bioactmat.2023.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]