FIG. 3.
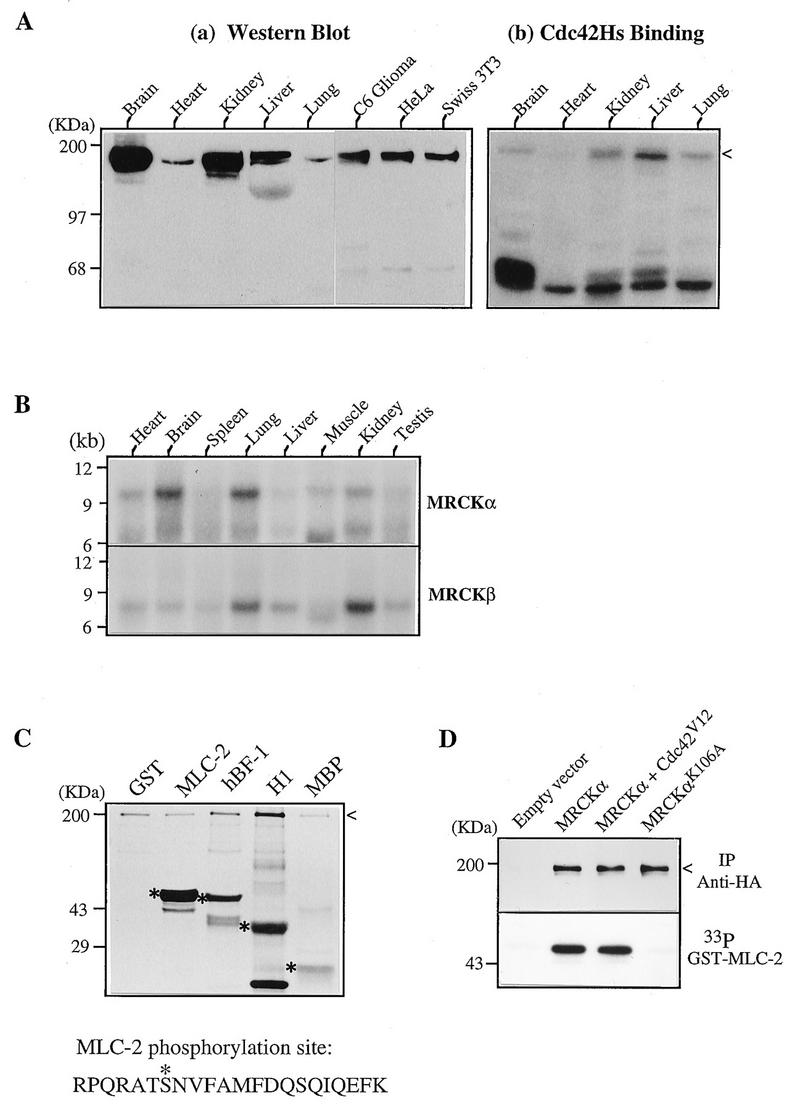
Expression and biochemical characterization of MRCKs. (A) Expression of MRCK in tissues and cultured cells. (a) Soluble protein extracts from various rat tissues and cells were separated by polyacrylamide gel electrophoresis and transferred to nitrocellulose filters for Western analysis using antibodies against the Cdc42-binding domain of human MRCKα (C-terminal 124 residues). (b) A similar blot showing Cdc42 binding. The arrowhead indicates the positions of the immunoreactive and Cdc42-binding regions. (B) Northern (mRNA) blot from rat tissues (Clontech) hybridized to the 32P-labeled MRCKα and MRCKβ cDNA probes. (C) Kinase activity toward different substrates. GST–MLC-2, GST-MRCKα p21-binding domain hBF-1 (residues 1 to 124; Fig. 1), histone H1, and myelin basic protein (MBP) were used as substrates in a kinase assay with purified MRCKα expressed in baculovirus as a GST fusion protein. The 33P-labeled bands corresponding to the Coomassie blue-stained substrate proteins are marked with asterisks, and the autophosphorylated GST-MRCKα band is indicated by an arrowhead. The sequence at the bottom shows the Lys-C peptide with the serine 19 (asterisk) phosphorylation site in MLC-2. (D) Kinase activities in transfected cells. COS-7 cells were transfected with vector pXJ40HA or with a vector containing MRCKα alone, MRCKα in combination with Cdc42V12, or kinase-inactive MRCKαK106A. Tagged proteins were immunoprecipitated (IP) with anti-HA antibody, and kinase activity (lower panel) was assayed with [γ-33P]ATP as described elsewhere (36).
