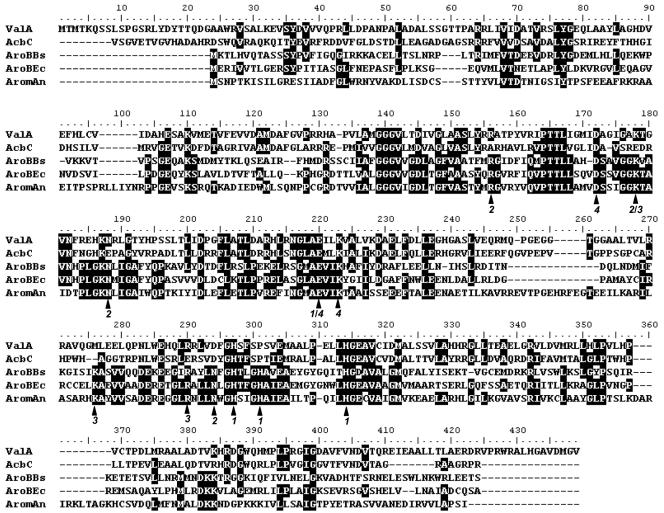
FIG. 4.
Alignment of ValA with AcbC and three AroB proteins. Deduced amino acid sequences are from the following organisms: AcbC, Actinoplanes sp. (Y18523.3); AroBBs, Bacillus subtilis (M80245); AroBEc, E. coli (X03867); and AromAn, Emericella (formerly Aspergillus) nidulans (395-amino-acid DHQS domain at the N terminus of the pentafunctional AROM protein; X05204). A functional attribution of the amino acid residues indicated with black arrows, based on the analysis of the three-dimensional structure of the dehydroquinate synthase (DHQS) domain of E. nidulans (4), is given below the alignment by the following code: 1 = Co2+ binding (Zn2+ in the fungal protein instead); 2 = heptulose phosphate group binding; 3 = C-1 hydroxyl fixation; and 4 = C-4 hydroxyl fixation.