Abstract
Volatile thiols, particularly 4-mercapto-4-methylpentan-2-one (4MMP), make an important contribution to the aroma of wine. During wine fermentation, Saccharomyces cerevisiae mediates the cleavage of a nonvolatile cysteinylated precursor in grape juice (Cys-4MMP) to release the volatile thiol 4MMP. Carbon-sulfur lyases are anticipated to be involved in this reaction. To establish the mechanism of 4MMP release and to develop strains that modulate its release, the effect of deleting genes encoding putative yeast carbon-sulfur lyases on the cleavage of Cys-4MMP was tested. The results led to the identification of four genes that influence the release of the volatile thiol 4MMP in a laboratory strain, indicating that the mechanism of release involves multiple genes. Deletion of the same genes from a homozygous derivative of the commercial wine yeast VL3 confirmed the importance of these genes in affecting 4MMP release. A strain deleted in a putative carbon-sulfur lyase gene, YAL012W, produced a second sulfur compound at significantly higher concentrations than those produced by the wild-type strain. Using mass spectrometry, this compound was identified as 2-methyltetrathiophen-3-one (MTHT), which was previously shown to contribute to wine aroma but was of unknown biosynthetic origin. The formation of MTHT in YAL012W deletion strains indicates a yeast biosynthetic origin of MTHT. The results demonstrate that the mechanism of synthesis of yeast-derived wine aroma components, even those present in small concentrations, can be investigated using genetic screens.
Volatile thiols are important aroma components of certain wine varieties, such as Sauvignon blanc and Scheurebe (16, 37), and have been isolated from wines made with many different cultivars of Vitis vinifera, including Gewürztraminer, Riesling, Colombard, Petit manseng, Semillon, Cabernet sauvignon, and Merlot (3, 26, 35, 37, 38). The first of the volatile thiols to be described was 4-mercapto-4-methylpentan-2-one (4MMP) (16, 17) (Fig. 1a), with aroma descriptions of “blackcurrant” and “broom tree” as well as “cats' urine” (at higher concentrations) (11, 17, 28). The thiol 4MMP has the lowest sensory detection threshold of any yeast metabolite described (14, 29), with reported values being 0.1 ng liter−1 and 3 ng liter−1 in water and wine, respectively. The concentrations found in wine, up to 30 ng liter−1 (11, 35), indicate its importance. In Scheurebe wine, 4MMP was the most important varietal odorant of the 42 compounds identified (16).
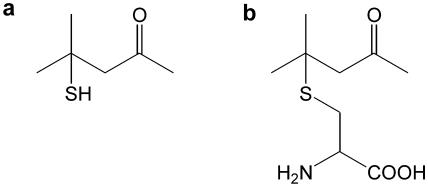
FIG. 1.
(a) Structure of volatile 4MMP. (b) Structure of Cys-4MMP.
The absence of volatile thiols in grapes indicates the importance of yeast fermentation in their formation. A cysteine-conjugated form of thiols has been proposed as the precursor present in grapes (35) (Fig. 1b). The metabolic activity of yeasts is necessary to cleave the cysteine-conjugated precursor (35), and fermentation with different wine yeast strains results in the release of different amounts of the volatile thiols (18, 26). Another volatile thiol present in wine, 3-mercaptohexanol (3MH), also originates from a cysteine-conjugated precursor (Cys-3MH). Studies of the formation of the free thiol have shown that a bacterial extract from Eubacterium limosum or purified tryptophanase from Escherichia coli cleaves the Cys-3MH precursor in vitro (39, 43). The extract from E. limosum and purified tryptophanase from E. coli exhibit cysteine-S-conjugate β-lyase activity. The wine yeast Saccharomyces cerevisiae is proposed to cleave thiol precursors during grape juice fermentation by a similar mechanism (39). Cysteine-S-conjugate lyases are part of a large enzyme family, the carbon-sulfur lyases, in which a carbon-sulfur bond is cleaved in a β-elimination reaction. Enzymes of this type have been isolated from rats, humans, zebra fish, and Arabidopsis thaliana (10, 23). It has been shown that β-lyase enzyme activity results in the formation of a free thiol and an intermediate that spontaneously degrades to pyruvate and ammonia (12). Therefore, it has been proposed that a yeast carbon-sulfur lyase with β-elimination activity could be involved in the release of 4MMP from Cys-4MMP.
The presence of aroma compounds bound as cysteine conjugates has been described for onion, garlic, and passion fruit (21, 36, 41). Cysteine conjugates have also been shown to exist in animals, and the release of cysteine-conjugated xenobiotics by β-lyases confirms the presence of these enzymes in higher eukaryotes (9). Moreover, enterobacteria such as E. limosum can cleave conjugated compounds, generating a toxic effect on the host animal (21, 23, 40). The description and sequencing of cysteine conjugates cleaving enzymes in bacteria and animals permit database searching for homologous enzymes in yeast.
Volatile compounds produced by yeast during alcoholic fermentation are particularly important for the aroma of wine (14). These aroma compounds can originate from distinct enzymatic reactions from well-characterized metabolic pathways in yeast and can be manipulated by expression of the enzymes involved, which in turn impacts the character of the resulting wine (13, 24, 29, 32, 33). However, many compounds of unknown biosynthetic origin arise during yeast fermentation (reviewed in references 5, 14, 22, and 29).
In a consumer-led wine industry, it is increasingly important to consistently produce wine to definable specifications and styles. To control and modulate the infinite number of flavor profiles of bottled wines, it is pivotal to understand the fundamentals of the synergies between grape varieties and flavor-active yeast strains. Such studies could, for example, offer prospects for the development of wine yeast starter strains with optimized volatile-thiol release.
The purpose of the present study was to investigate yeast's mechanism of volatile-thiol release by using a genetic strategy. Potential carbon-sulfur lyase genes, identified using protein sequence comparisons and database searching, were deleted from laboratory and wine yeast strains. The possible role of these genes in thiol release was then evaluated by performing fermentations in a medium with Cys-4MMP.
This study demonstrates that genes necessary for the synthesis of wine aroma components, even those present in small concentrations, can be identified by using a combination of genetic screens and advanced analytical chemistry. The results indicate that more than one enzyme encoded by the yeast genome plays a role in volatile-thiol release. These findings offer prospects for the development of wine yeast starter strains with optimized volatile-thiol release capabilities that could assist winemakers in their efforts to consistently produce wine to definable flavor specifications and styles.
MATERIALS AND METHODS
Reagents, media, and fermentation conditions.
Analytical reagents were purchased from Sigma-Aldrich (Castle Hill, Australia). Cys-4MMP was synthesized as previously described (18) (Fig. 1b). Medium constituents and molecular biology reagents were purchased from Difco (Detroit, MI) and Sigma-Aldrich, respectively, unless stated otherwise. Yeast strains were grown in a synthetic medium (2) consisting of 0.67% yeast nitrogen base (Difco), an amino acid mix, and 8% d-glucose at 28°C with shaking at 200 rpm. Cys-4MMP was added to a final concentration of 0.1 mg ml−1. Fermentations were conducted in 250-ml conical flasks fitted with a water lock and side arm septum for sampling. Triplicate starter cultures of yeasts were prepared in YPD (yeast extract [10 g liter−1], peptone [20 g liter−1], and d-glucose [20 g liter−1]), grown to stationary phase, and diluted 100-fold for inoculation. Each yeast strain was grown in triplicate at 18°C, and the cultures were sampled regularly for cell counts (measured spectrophotometrically at 600 nm) and sugar concentrations (refractive indexes). Completed fermentations (after 40 h) were clarified by centrifugation (4,000 rpm for 5 min), and the supernatants were stored at −20°C until the time of analysis.
Yeast strains.
The wine yeast strain S. cerevisiae VL3 was sourced from the culture collection of the Australian Wine Research Institute (Adelaide, Australia). Haploid (matα) S. cerevisiae yeast (BY4742) was used as the wild-type laboratory reference strain. Deletion strains in this background were selected from the Euroscarf deletion collection (http://www-sequence.stanford.edu) (Table 1).
TABLE 1.
S. cerevisiae strains used for this study
| Strain | Genotype | Source |
|---|---|---|
| BY4742 | BY4741 MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Euroscarf |
| VL3 | Isolate from commercial wine yeast VL3, Lafforte Oenology | AWRI culture collection |
| VL3p | Sporulated and dissected VL3 | This study |
| VLc | Sporulated and dissected VL3p | This study |
| VLd | Sporulated and dissected VL3p | This study |
| BY4742 (YJL060W) | BY4742 MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 YJL060WΔ::KanMX4 | Euroscarf |
| BY4742 (YAL012W) | BY4742 MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 YAL012WΔ::KanMX4 | Euroscarf |
| BY4742 (YFR055W) | BY4742 MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 YFR055WΔ::KanMX4 | Euroscarf |
| BY4742 (YML004C) | BY4742 MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 YML004CΔ::KanMX4 | Euroscarf |
| AWRI 04 | VLc YJL060WΔ::KanMX4/YJL060WΔ::KanMX4 | This study |
| AWRI 05 | VLc YFR055WΔ::KanMX4/YFR055WΔ::KanMX4 | This study |
| AWRI 06 | VLc YAL012WΔ::KanMX4/YAL012WΔ::KanMX4 | This study |
Database searching.
S. cerevisiae genes with putative C-S lyase activity were identified in the BRENDA protein database (EC 4.4.*.*) (www.brenda.uni-koeln.de). Multiple alignments were performed using the program ClustalW at the European Bioinformatics Institute website (http://www.ebi.ac.uk/clustalw/) and three protein sequences with known C-S lyase activity from humans, rats, and zebra fish, with respective accession numbers of NP_004050, AAB26845, and CAB44334. A C-S lyase consensus sequence was identified with the program Pretty (http://www-igbmc.u-strasbg.fr/BioInfo). The consensus sequence was aligned with the S. cerevisiae database in a BLAST search at the Saccharomyces Genome Database home page (http://www.yeastgenome.org).
Analytical chemistry of sulfur compounds.
Gas chromatography coupled to atomic emission detection (GC-AED) was carried out on a Hewlett Packard 5890 series II gas chromatograph coupled to a G2350A atomic emission detector (Hewlett Packard), as described by Howell et al. (18). Following the methods of Mestres et al. (25), 0.1 g EDTA and 2 g NaCl were added to 10 ml of sample in a 15-ml sample vial. The internal standard (propyl thioacetate [0.1 μg l−1]) was injected through the septum. The vials were placed in a 45°C water bath, and the fiber was exposed for 45 min before injection into the gas chromatograph. Fermentation samples were appropriately diluted to fit the calibration range and divided into aliquots in duplicate for analysis.
GC and sample preparation for GC-mass spectrometry (GC-MS) analysis were performed as described above for GC-AED analysis. The mass spectrometer used was an HP5973 spectrometer. Positive-ion impact spectra at 70 eV were recorded in the range of m/z 50 to 300 for scan runs.
Construction of gene deletions in a wine yeast derivative.
Yeasts were sporulated according to the method of Brachmann et al. (7), with slight modifications. Briefly, the cells were streaked onto fresh sporulation medium for two overnight periods, and a generous match head of cell biomass was placed into 2 ml potassium acetate-lithium acetate medium and incubated at 25°C with shaking until asci were observed (3 to 4 days).
The cells were prepared for dissection according to the method of Burke et al. (8). The sporulated culture (1 ml) was pulse centrifuged, and the pellet was resuspended in 50 μl of 10-mg liter−1 Zymolyase (in 1 M sorbitol) and incubated at 30°C for 20 min. The cells were then transferred to a fresh YPD plate, and asci were microdissected with an Olympus CHT microscope modified for microdissection.
Genes were deleted by a PCR-mediated gene disruption technique described by Wach et al. (4, 42). Primers were designed for each of the target genes, with up to 500-bp flanking regions on either end (see Table 3). Primers were obtained desalted from Geneworks (Adelaide, Australia). Genomic DNA was prepared by the method of Ausubel et al. (2), where a breaking buffer and glass beads are used to break open the cells with a Mini-Beadbeater 8 (Biospec Products). To amplify the disruption cassettes of interest, PCR was performed on DNA prepared from the respective deletion strain, and this cassette was used in transformation reactions. The PCR (100 μl) mixture contained PCR buffer with MgCl2 at 1.5 mM (Advanced Biotechnologies) (1×), deoxynucleoside triphosphates (a 200 μM concentration of each), primers (a 0.5 μM concentration of each), 1.5 mM Taq polymerase (Advanced Biotechnologies) (1.25 U), and template DNA (40 ng). The reactions were performed in an MJ Research PTC-100 programmable thermal cycler programmed as follows: denaturation of DNA at 94°C for 2 min followed by 30 cycles of 30 s of denaturation of DNA at 94°C, 1 min of primer annealing at 53°C, and 1 min of DNA extension at 72°C. The final extension period was 10 min, and the tubes were then cooled to 4°C. The PCR products were run in a 1.2% (wt/vol) agarose gel (Scientifix) and stained with ethidium bromide to visualize the DNA to ensure correct amplification. Amplification reaction mixtures were ethanol precipitated, and the concentrations were determined on a gel (2). S. cerevisiae laboratory strain BY4742 and S. cerevisiae yeast strain VLc were transformed by a lithium acetate-polyethylene glycol method (2) with up to 5 μg of DNA. After growth in YPD for 2 h at 30°C, transformants were selected on YPD (containing G418 sulfate at 100 μg liter−1 [for lab yeast] or 300 μg liter−1 [for wine yeast]) and grown at 30°C for 2 days or until colonies appeared. Transformants that had undergone homologous recombination were tested by replica plating onto selective YPD (G418) medium. After sporulation and microdissection, genomic DNAs were prepared from viable spores as described above. PCR was used to confirm the presence of the deletion cassette by amplification with the forward and reverse primers used to amplify the fragment.
TABLE 3.
Primers for construction and confirmation of deletion strains
| Primer namea | Sequence (5′-3′) |
|---|---|
| YJL-F | TGTTTAAAAGGGGAAGGTACGA |
| YJL-R | CGCGAAGAAAATATGGAATTA |
| YAL-F | ACGAGATTAGCGACCTCGAA |
| YAL-R | CGCATGCATATGCCTTTATG |
| YFR-F | CGATTTTATTGTCGCCTCTTG |
| YFR-R | TCATTTCCGCTGAATAAGTACG |
| KanMX4-R | CGACTGAATCCGGTGAGAAT |
F and R, forward and reverse primers, respectively.
RESULTS
Identification of C-S lyases involved in release of 4MMP.
The release of the volatile thiol 4MMP from its grape-derived cysteine-bound precursor is dependent on yeast (18, 39). To screen for genes that cleave the carbon-sulfur bond of the cysteine precursor of 4MMP, strains possessing deletions in genes of interest were selected. S. cerevisiae sequences with similarity to human, rat, and zebra fish carbon-sulfur lyases were identified using BLAST searches (see Materials and Methods). Using this method, locus YJL060W was identified as a candidate for cysteine conjugate β-lyase activity on Cys-4MMP. Other candidate genes, YML004C (GLO1), CYS3 (YAL012W), and YFR055C, encode enzymes with previously shown or predicted carbon-sulfur lyase activity.
The YJL060W gene is annotated in the Saccharomyces Genome Database as encoding a putative aminotransferase based on a pyridoxal phosphate binding domain and an aminotransferase family I region. Recent work has implicated YJL060W in the biosynthesis of nicotinic acid (encoded by BNA3), and it has been hypothesized to be an arylformidase (27).
A search of the BRENDA enzyme database revealed genes encoding enzymes that could cleave carbon-sulfur bonds in S. cerevisiae (EC 4.4.1.*) (Table 2). The CYS3 (YAL012W) gene encodes cystathionine γ-lyase (EC 4.4.1.1) and shows 26% similarity to the YJL060W gene. The Cys3 protein catalyzes the reaction of cystathionine to cysteine in the trans-sulfuration pathway to interconvert cysteine and methionine (34). A gene that shows similarity to cystathionine β-lyase, YFR055C, has been inferred by structure to encode the enzyme that converts cystathionine to homocysteine, pyruvate, and ammonia (EC 4.4.1.8) (34). S. cerevisiae has been shown to have an enzymatic activity of this type, but no gene has been specified (9). The final carbon-sulfur lyase described for S. cerevisiae is lactoylglutathione lyase (EC 4.4.1.5), encoded by the gene GLO1 (YML004C). The Glo1 protein catalyzes the conjugation of methylglyoxal to glutathionine as a detoxification mechanism from the consequences of the toxic metabolite formed during glycolysis (19). The enzyme Glo1p has also been shown to act on an intermediate of glutathionine biosynthesis, γ-glutamylcysteine (19), indicating that this enzyme could have a broad specificity.
TABLE 2.
Genes identified as having potential carbon-sulfur lyase activity on the cysteinylated 4MMP precursor, Cys-4MMP
| Systematic name | Gene name | Gene function | EC no. | Comment |
|---|---|---|---|---|
| YJL060W | BNA3 | Biosynthesis of nicotinic acid | Similar to human/rat cysteine conjugate lyases | |
| YML004C | GLO1 | Glyoxalase 1, lactoylglutathione lyase | 4.4.1.5 | Yeast C-S lyase |
| YFR055W | METC | Cystathionine beta-lyase | 4.4.1.8 | Yeast C-S lyase |
| YAL012W | CYS3 | Cystathionine gamma-lyase | 4.4.1.1 | 26% similar to YFR055W, 26% similar to YLR303W |
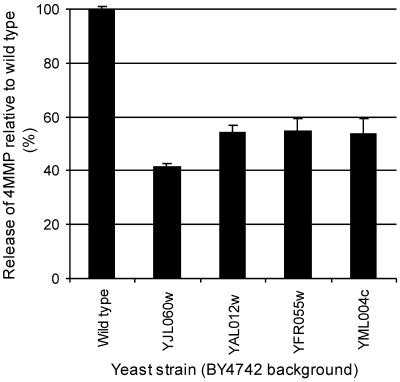
To investigate whether these genes have a role in the formation of 4MMP, fermentations with the respective deletion strains were conducted in defined media with the 4MMP precursor, Cys-4MMP. Growth was monitored by optical density measurements at 600 nm, and all of the strains grew at the same rate (data not shown). Fermentation with BY4742 (YJL060W deletion strain) resulted in a 41% reduction in 4MMP release compared to that of the wild-type yeast strain BY4742 (Fig. 2). Deletions in genes YAL012W, YFR055C, and YML004C in the BY4742 background also reduced the amount of 4MMP released, to 53 to 54% that of the control (Fig. 2).
FIG. 2.
Deletions in genes encoding predicted carbon-sulfur lyases affect the release of 4MMP from a laboratory strain (BY4742) of Saccharomyces cerevisiae. The amounts of 4MMP measured at the end of fermentations with S. cerevisiae deletion strains are expressed as percentages of the amount of 4MMP released by the wild-type laboratory strain BY4742 (wild-type BY4742 release = 0.23 ± 0.06 μg/ml). Gene names correspond to deletions of those genes from the laboratory strain background. Results show the means of triplicate fermentations, and bars give standard errors of the means.
Construction and confirmation of deletions in wine yeast.
The wild-type laboratory yeast strain BY4742 released only 0.5 to 1.2% molar equivalents of the Cys-4MMP precursor added. The low level of 4MMP release was accurately measured by the solid-phase microextraction-GC-AED method, yet the molar release of 4MMP was much lower than that seen with wine yeast strains of S. cerevisiae (18). To investigate the involvement of the deleted genes in the release of 4MMP in a wine yeast genetic background, deletion strains were constructed in a derivative of the wine yeast S. cerevisiae VL3. Strain VL3 has been shown previously to release large amounts of 4MMP (18, 26).
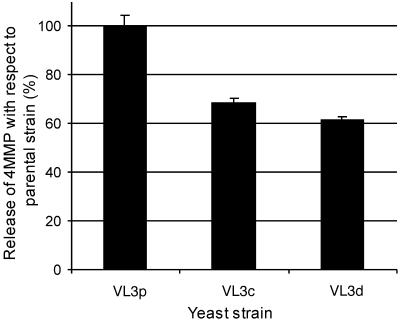
Wine yeast strains are generally considered to be heterozygous and aneuploid or polyploid (15). Additionally, stable haploids cannot be produced because of a functional HO gene. To reduce the complexity of the genetic background and to produce homozygous diploids, the parental strain VL3p was sporulated and dissected to produce VL3a, -b, -c, and -d. These sporulants rapidly rediploidized to show a Mata/Matα genotype by PCR (data not shown), and these VL3-derived strains were tested for the ability to release 4MMP and to produce viable spores. VL3a and -b did not sporulate well and were discarded. VL3c and VL3d released 4MMP effectively, although the amount was reduced with respect to that from the parental strain VL3p (Fig. 3). VL3c, which sporulated efficiently and produced viable spores, was used as the homozygous diploid for further deletions. A deletion cassette containing the KanMX4 gene and homologous flanking regions for the gene of interest was amplified by PCR using the primers indicated in Table 3 and was used to transform VL3c. Geneticin-resistant transformants were sporulated and dissected, and the spores were retested for Geneticin resistance and replacement of the gene with KanMX4 by PCR (data not shown). We were unable to obtain Geneticin-resistant yeast with a deletion cassette for YML004C.
FIG. 3.
Sporulant strains derived from a commercial wine yeast, Saccharomyces cerevisiae VL3, release less 4MMP than the parental VL3 strain. Sporulants from VL3 were fermented with Cys-4MMP, and the amount of 4MMP was measured by GC-AED. The amount of 4MMP released is expressed relative to that released by the wild-type yeast VL3p (wild-type VL3p release = 950.36 ± 13.75 μg/ml), and bars give standard errors of the means.
When the YAL012W gene was deleted in VL3c, a growth defect was observed, which was overcome by supplementation of the medium with cysteine. BY4742 (YAL012W deletion strain) is a cysteine auxotroph, since the pathway to convert methionine to cysteine is rendered nonfunctional without cystathionine γ-lyase (34). Additionally, when genes YJL060W and YFR055C were deleted in VL3c, no difference in growth was observed with respect to that of the wild type.
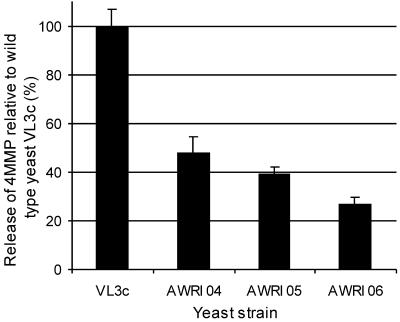
Transformants with 2:2 segregation of the wild-type gene from the deleted gene were then fermented with Cys-4MMP, and the amount of 4MMP produced was measured. The deletion of gene YJL060W from strain AWRI 04 reduced the amount of 4MMP measured to 48% that from wild-type VL3c (Fig. 4). The deletion of YAL012W in the wine yeast background (strain AWRI 05) resulted in a reduction of 4MMP to 39% of that measured in the wild-type strain (Fig. 4). When YFR055W was deleted from VL3c, giving strain AWRI 06, a reduction in the amount of 4MMP measured was also found (27% of the wild-type release) (Fig. 4). These data confirm that these genes play a role in the release of 4MMP from a wine yeast, although with different effects than in the laboratory yeast background.
FIG. 4.
Saccharomyces cerevisiae wine yeast strains deleted in genes YAL012W, YFR055C, and YJL060W (AWRI 04, AWRI 05, and AWRI 06, respectively) have reduced 4MMP release during fermentation. A sporulant, VL3c, from the widely used VL3 commercial wine yeast was transformed with the YAL012W, YFR055C, and YJL060W KanMX deletion cassettes derived from the BY4742 deletion library. Positive transformants were sporulated. The four spores from one tetrad showing 2:2 segregation of wild-type and deletion genes were fermented with Cys-4MMP. The amount of 4MMP was measured by GC-AED. The amount of 4MMP released is expressed relative to that released by the wild-type yeast (VL3c), and bars give standard errors of the means.
MTHT, a novel yeast metabolite.
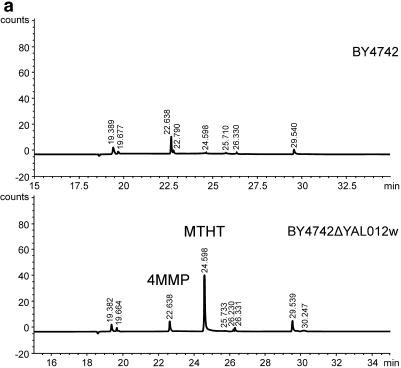
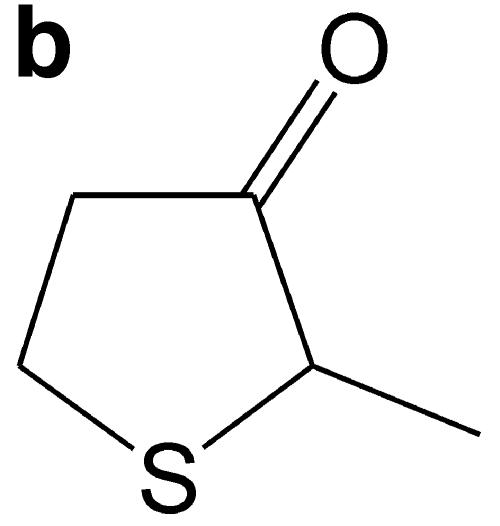
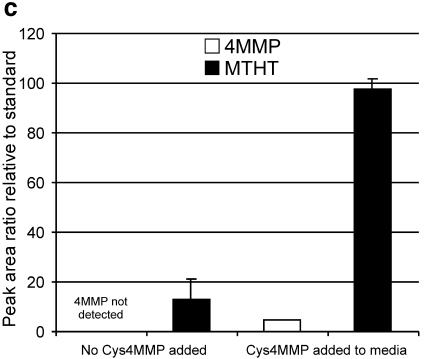
The release of 4MMP was measured using sulfur-specific acquisition for GC-AED. When the volatiles from the fermentation with yeast BY4742 (YAL012W deletion strain) were analyzed, an additional compound eluted at 24 min (Fig. 5a). A peak with the same retention time was also present in fermentations with wild-type BY4742, but at a lower intensity. Library searches of the mass fragment pattern by GC-MS identified this peak as 2-methyltetrathiophen-3-one (MTHT) (Fig. 5b). The identity of the compound was confirmed with authentic 2-methyltetrathiophen-3-one, which had the same retention time and fragmentation pattern as the peak observed on the GC-AED trace (data not shown). The amount of MTHT released was dependent on the presence of Cys-4MMP in the medium (Fig. 5c). 4MMP was not detected in fermentations without Cys-4MMP, where MTHT was present in small amounts. The addition of Cys-4MMP increased the amount of detectable MTHT in the medium 6.5-fold.
FIG. 5.
(a) GC-AED chromatograms from fermentations conducted with a wild-type laboratory strain of Saccharomyces cerevisiae, BY4742, and the BY4742 strain carrying a deletion of the YAL012W gene. Chromatograms were obtained by monitoring at 181 nm (sulfur acquisition). The propyl thioacetate internal standard elutes at 19.22 min, 4MMP elutes at 22.63 min, and the peak subsequently identified by GC-MS as MTHT elutes at 24.59 min. (b) Structure of MTHT. (c) Release of MTHT is dependent on the presence of Cys-4MMP in the fermentation medium. Cys-4MMP was added to triplicate fermentations of BY4742 (YAL012W deletion strain). The amounts of 4MMP and MTHT released were quantified relative to an internal standard, propyl thioacetate (see Materials and Methods). Bars give standard errors of the means.
DISCUSSION
Although S. cerevisiae was the first eukaryotic organism to have its genome sequenced, with the full sequence becoming available in 1996, there have been few studies on the effects of genes on the formation of specific aroma metabolites (for examples, see references 1 and 13). The yeast S. cerevisiae is the primary organism used to make wine. The yeast converts sugars to ethanol and carbon dioxide, and importantly, produces aroma compounds that impact the sensory characteristics of the finished product. Searching for genes that affect the metabolic profile could reveal genes that encode proteins involved in aroma release and provide information on their function. In this study, genes with a predicted role in sulfur metabolism were studied, with a specific focus on the release of volatile thiols.
The volatile thiols are influential odorants for a wide range of wine styles (3, 26, 35, 37). Yeast acts upon nonvolatile cysteine conjugate precursors to release the thiols in small concentrations (ng liter−1) to produce the distinct aromas described for the compounds (38). It is believed that this is carried out by enzymes that possess carbon-sulfur lyase activity. Carbon-sulfur lyases in yeast that potentially cleave the nonvolatile cysteine conjugate in grapes to form the volatile thiol in wine were identified by examining enzyme and protein databases. Also, DNA comparisons revealed an S. cerevisiae gene similar to cysteine conjugate β-lyases found in humans and rats.
The open reading frame YJL060W was a candidate for volatile-thiol release, with the encoded protein sequence having similarity to known carbon-sulfur lyases. The deletion of YJL060W reduced 4MMP release in laboratory and wine yeast backgrounds. Although it is described as an arylformidase (27), the function has not been confirmed for YJL060W. The predicted sequence of the protein encoded by YJL060W contains a pyridoxal 5′ phosphate binding site and a putative aminotransferase region. The functions of YJL060W in wine aroma elaboration and in general metabolism are being investigated.
The carbon-sulfur lyase genes identified thus far in S. cerevisiae encode enzymes of the cysteine and methionine interconversion pathway. CYS3 encodes cystathionine β-lyase, which converts cystathionine to give cysteine, pyruvate, and ammonia. The gene product of the open reading frame YFR055W catalyzes the reverse reaction of cystathionine to homocysteine, pyruvate, and ammonia and has a 40% predicted amino acid similarity to cystathionine β-lyase (34). The deletion of either of these genes in the laboratory yeast and wine yeast backgrounds also resulted in a reduction in 4MMP release. Similarly, the deletion of YML004C, the GLO1 gene in S. cerevisiae, decreased the amount of 4MMP in the medium. GLO1 is involved in central metabolism homeostasis (20). These results demonstrate that the release of 4MMP is mediated by protein products of multiple genes in S. cerevisiae. A direct role of these enzymes in the cleavage of Cys-4MMP still needs to be confirmed.
Differential expression of the genes identified in this study does not explain the ability of different wine yeast strains to release 4MMP. Previous work in our laboratory reported that wine yeast strains used for the commercial production of wine show variability in the ability to release 4MMP. However, preliminary studies indicated that there are no clear differences in relative transcript abundance between wine yeasts that produce large amounts of 4MMP and wine yeasts that produce only small amounts (data not shown). These results support the hypothesis that several gene products are involved in the release of the volatile thiol 4MMP and that a combination of gene products confers the ability of wine yeasts to release differing amounts of 4MMP. The presence of a wine yeast gene product which has a higher activity on Cys-4MMP may exist and cannot be excluded until a larger collection of deletion strains is screened and the genome sequences of wine yeast strains are available. Moreover, the main control point of thiol release in wine yeast strains might not be at the level of Cys-4MMP cleavage. Transport of the cysteine conjugate into the cell, for example, may contribute to the release of 4MMP. Interestingly, the transcript levels of the genes CYS3 and YFR055W are upregulated in response to sulfur starvation (6), and GLO1 is upregulated in response to osmotic stress (20). Sulfur starvation and osmotic stress are frequently experienced by yeast in wine fermentations (31) but were unlikely to be factors in the experiments described here.
In the present study, metabolic differences were detected by the sulfur-selective acquisition of GC-AED, which has identified another compound with relevance to wine aroma (MTHT). The biosynthetic origin of MTHT remains unclear. It could be derived from a differential breakdown of Cys-4MMP or from a less direct mechanism, such as the circularization of 4MMP itself. The observation that the MTHT concentration increases in the BY4742 (YAL012W) deletion strain is most easily explained by MTHT being an intermediate in the metabolism of Cys-4MMP or 4MMP and by the fact that further reaction requires the YAL012W gene product. It cannot be ruled out, however, that the loss of the YAL012W gene increases the expression of other proteins that are involved in MTHT synthesis. Purification of the corresponding enzyme and an in vitro assay for MTHT synthesis from Cys-4MMP and 4MMP will be necessary to confirm the origin of this potentially important sulfur metabolite.
Our approach to establishing which genes are involved in the release of 4MMP is different from techniques previously used to define effectors of wine aroma. A concurrent approach to genetic studies of wine aroma detection is a metabolomic footprinting approach that categorizes deletions based on the extracellular medium profile by liquid chromatography-MS (1, 30) or GC-MS (13). Extracellular medium profiling is useful for wine aroma determination, as it is yeast metabolites which determine the aromas formed in a wine. The present work has studied a specific group of yeast metabolites, the sulfur compounds, which are known to strongly impact wine aroma. The metabolic derivations of sulfur compounds are unknown, so a genetic approach was used to elucidate their yeast origins.
The elucidation of the biosynthetic origins of new and potent aroma compounds can be performed by bioinformatics to predict genes. We have shown by this method that deletions in genes which can cleave or are hypothesized to cleave a carbon-sulfur bond can affect the release of the volatile thiol 4MMP. The power of such a technique is shown by the identification of MTHT, an important wine aroma compound with a previously unknown origin. Gene deletion studies can therefore elucidate the origins of new compounds, even those present at small concentrations.
Acknowledgments
Sonia Dayan provided excellent advice and assistance for the planning and execution of real-time PCR.
This project was supported by Australia's grapegrowers and winemakers through their investment body, the Grape and Wine Research Development Corporation, with matching funds from the Australian Government. K.S.H. is the recipient of an Australian Postgraduate Award stipend and an AWRI scholarship.
REFERENCES
- 1.Allen, J., H. M. Davey, D. Broadhurst, J. K. Heald, J. J. Rowland, S. G. Oliver, and D. B. Kell. 2003. High-throughput classification of yeast mutants for functional genomics using metabolic footprinting. Nat. Biotechnol. 21:692-696. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, and R. E. Kingston (ed.). 1994. Current protocols in molecular biology, p. 13.0.1-13.14.17. Wiley, New York, N.Y.
- 3.Aznar, M., R. Lopez, J. F. Cacho, and V. Ferreira. 2001. Identification and quantification of impact odorants of aged red wines from Rioja. GC-olfactometry, quantitative GC-MS, and odor evaluation of HPLC fractions. J. Agric. Food Chem. 49:2924-2929. [DOI] [PubMed] [Google Scholar]
- 4.Baudin, A., O. Ozier-Kalogeropoulos, A. Denouel, F. Lacroute, C. Cullin, A. Wach, A. Brachat, R. Pohlmann, and P. Philippsen. 1993. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae; new heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Nucleic Acids Res. 21:3329-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry, D. R., and D. C. Watson. 1987. Production of organoleptic compounds, p. 345-368. In D. R. Berry, I. Russell, and G. G. Stewart (ed.), Yeast biotechnology. Allen and Unwin London, London, United Kingdom.
- 6.Boer, V. M., J. H. de Winde, J. T. Pronk, and M. D. W. Piper. 2003. The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J. Biol. Chem. 278:3265-3274. [DOI] [PubMed] [Google Scholar]
- 7.Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li, P. Hieter, and J. D. Boeke. 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115-132. [DOI] [PubMed] [Google Scholar]
- 8.Burke, D., D. Dawson, and T. Stearns. 2000. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 9.Cherest, H., and Y. Surdin-Kerjan. 1992. Genetic analysis of a new mutation conferring cysteine auxotrophy in Saccharomyces cerevisiae: updating of the sulfur metabolism pathway. Genetics 130:51-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, A. J. L., S. A. Bruschi, and M. W. Anders. 2002. Toxic, halogenated cysteine S-conjugates and targeting of mitochondrial enzymes of energy metabolism. Biochem. Pharmacol. 64:553-564. [DOI] [PubMed] [Google Scholar]
- 11.Darriet, P., T. Tominga, V. Lavigne, J. Boidron, and D. Dubourdieu. 1995. Identification of a powerful aromatic compound of Vitis vinifera L. var. Sauvignon wines: 4-mercapto-4-methylpentan-2-one. Flavour Fragrance J. 10:385-392. [Google Scholar]
- 12.Davis, L., and D. E. Metzler. 1972. Pyridoxal-linked elimination and replacement reactions, p. 33-74. In P. D. Boyer (ed.), The enzymes, 3rd ed., vol. 7. Academic Press, New York, N.Y. [Google Scholar]
- 13.Eglinton, J. M., A. J. Heinrich, A. P. Pollnitz, P. Langridge, P. A. Henschke, and M. de Barros Lopes. 2002. Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldehyde dehydrogenase gene. Yeast 19:295-301. [DOI] [PubMed] [Google Scholar]
- 14.Etievant, P. X. 1991. Wine, p. 483-546. In H. Maarse (ed.), Volatile compounds in foods and beverages. Marcel Dekker Inc., New York, N.Y.
- 15.Guijo, S., J. C. Mauricio, J. M. Salmon, and J. M. Ortega. 1997. Determination of the relative ploidy in different Saccharomyces cerevisiae strains used for fermentation and ‘flor’ film ageing of dry sherry-type wines. Yeast 13:101-117. [DOI] [PubMed] [Google Scholar]
- 16.Guth, H. 1997. Identification of character impact odorants of different white wine varieties. J. Agric. Food Chem. 45:3022-3026. [Google Scholar]
- 17.Guth, H. 1997. Quantification and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 45:3027-3032. [DOI] [PubMed] [Google Scholar]
- 18.Howell, K. S., J. H. Swiegers, G. Elsey, T. E. Siebert, E. J. Bartowsky, G. H. Fleet, Pretorius, I. S., and M. de Barros Lopes. 2004. Variation in 4-mercapto-4-methyl-pentan-2-one release by different wine strains. FEMS Microbiol. Lett. 240:125-129. [DOI] [PubMed] [Google Scholar]
- 19.Inoue, Y., and A. Kimura. 1996. Identification of the structural gene for glyoxalase I from Saccharomyces cerevisiae. J. Biol. Chem. 271:25958-25965. [PubMed] [Google Scholar]
- 20.Inoue, Y., Y. Tsujimoto, and A. Kimura. 1998. Expression of the glyoxalase I gene of Saccharomyces cerevisiae is regulated by high osmolarity glycerol mitogen-activated protein kinase pathway in osmotic stress response. J. Biol. Chem. 273:2977-2983. [DOI] [PubMed] [Google Scholar]
- 21.Keusgen, M., H. Schulz, J. Glodek, I. Krest, H. Kruger, N. Herchert, and J. Keller. 2002. Characterization of some Allium hybrids by aroma precursors, aroma profiles, and alliinase activity. J. Agric. Food Chem. 50:2884-2890. [DOI] [PubMed] [Google Scholar]
- 22.Lambrechts, M. G., and I. S. Pretorius. 2000. Yeast and its importance to wine aroma—a review. S. Afr. J. Enol. Vitic. 21:97-129. [Google Scholar]
- 23.Lash, L. H., R. M. Nelson, R. A. Van Dyke, and M. W. Anders. 1990. Purification and characterization of human kidney cytosolic cysteine conjugate beta-lyase activity. Drug Metab. Dispos. 18:50-54. [PubMed] [Google Scholar]
- 24.Lilly, M., M. G. Lambrechts, and I. S. Pretorius. 2000. Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Appl. Environ. Microbiol. 66:744-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mestres, M., C. Sala, M. P. Marti, O. Busto, and J. Guasch. 1999. Headspace solid-phase microextraction of sulphides and disulphides using carboxen-polydimethylsiloxane fibers in the analysis of wine aroma. J. Chromatogr. A 835:137-144. [DOI] [PubMed] [Google Scholar]
- 26.Murat, M. L., I. Masneuf, P. Darriet, V. Lavigne, T. Tominga, and D. Dubourdieu. 2001. Effect of Saccharomyces cerevisiae yeast strains on the liberation of volatile thiols in Sauvignon blanc wine. Am. J. Enol. Vitic. 52:136-139. [Google Scholar]
- 27.Panozzo, C., M. Nawara, C. Suski, R. Kucharczyka, M. Skoneczny, A. M. Becam, J. Rytka, and C. J. Herbert. 2002. Aerobic and anaerobic NAD+ metabolism in Saccharomyces cerevisiae. FEBS Lett. 517:97-102. [DOI] [PubMed] [Google Scholar]
- 28.Pearce, T. J. P., J. M. Peacock, F. Aylward, and D. R. Haisman. 1967. Catty odours in food: reactions between hydrogen sulphide and unsaturated ketones. Chem. Ind. (London) 37:1562-1563. [PubMed] [Google Scholar]
- 29.Pretorius, I. S. 2000. Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16:675-729. [DOI] [PubMed] [Google Scholar]
- 30.Raamsdonk, L. M., B. Teusink, D. Broadhurst, N. Zhang, A. Hayes, M. C. Walsh, J. A. Berden, K. M. Brindle, D. B. Kell, J. J. Rowland, H. V. Westerhoff, K. van Dam, and S. G. Oliver. 2001. A functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations. Nat. Biotechnol. 19:45-50. [DOI] [PubMed] [Google Scholar]
- 31.Rauhut, D. 1993. Yeasts—production of sulfur compounds, p. 183-223. In G. H. Fleet (ed.), Wine microbiology and biotechnology. Harwood Academic Publishers, Chur, Switzerland.
- 32.Remize, F., J. L. Roustan, J. M. Sablayrolles, P. Barre, and S. Dequin. 1999. Glycerol overproduction by engineered Saccharomyces cerevisiae wine yeast strains leads to substantial changes in by-product formation and to a stimulation of fermentation rate in stationary phase. Appl. Environ. Microbiol. 65:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiropoulos, A., and L. F. Bisson. 2000. MET17 and hydrogen sulfide formation in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 66:4421-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas, D., and Y. Surdin-Kerjan. 1997. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 61:503-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tominaga, T., R. Baltenweck-Guyot, C. Peyrot de Gachons, and D. Dubourdieu. 2000. Contribution of volatile thiols to the aromas of white wines made from several Vitis vinifera grape varieties. Am. J. Enol. Vitic. 51:178-181. [Google Scholar]
- 36.Tominaga, T., and D. Dubourdieu. 2000. Identification of cysteinylated aroma precursors of certain volatile thiols in passion fruit juice. J. Agric. Food Chem. 48:2874-2876. [DOI] [PubMed] [Google Scholar]
- 37.Tominaga, T., A. Furrer, R. Henry, and D. Dubourdieu. 1998. Identification of new volatile thiols in the aroma of Vitis vinifera L. var. Sauvignon blanc wines. Flavour Fragrance J. 13:159-162. [Google Scholar]
- 38.Tominaga, T., M. L. Murat, and D. Dubourdieu. 1998. Development of a method for analyzing the volatile thiols involved in the characteristic aroma of wines made from Vitis vinifera L. cv. Sauvignon blanc. J. Agric. Food Chem. 46:1044-1048. [Google Scholar]
- 39.Tominaga, T., C. Peyrot des Gachons, and D. Dubourdieu. 1998. A new type of flavour precursors in Vitis vinifera L. cv. Sauvignon blanc: S-cysteine conjugates. J. Agric. Food Chem. 46:5215-5219. [DOI] [PubMed] [Google Scholar]
- 40.Tomisawa, H., S. Suzuki, S. Ichihara, H. Fukazawa, and M. Tateishi. 1984. Purification and characterization of C-S lyase from Fusobacterium varium. A C-S cleavage enzyme of cysteine conjugates and some S-containing amino acids. J. Biol. Chem. 259:2588-2593. [PubMed] [Google Scholar]
- 41.Tsuge, K., M. Kataoka, and Y. Seto. 2002. Determination of S-methyl-, S-propyl-, and S-propenyl-l-cysteine sulfoxides by gas chromatography-mass spectrometry after tert-butyldimethylsilylation. J. Agric. Food Chem. 50:4445-4451. [DOI] [PubMed] [Google Scholar]
- 42.Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793-1808. [DOI] [PubMed] [Google Scholar]
- 43.Wakabayashi, H., M. Wakabayashi, W. Eisenreich, and K. H. Engel. 2004. Stereochemical course of the generation of 3-mercaptohexanal and 3-mercaptohexanol by beta-lyase-catalyzed cleavage of cysteine conjugates. J. Agric. Food Chem. 52:110-116. [DOI] [PubMed] [Google Scholar]